From Wikipedia, the free encyclopedia
4,5-MDO-DMT
Names
Preferred IUPAC name
2-(2H ,6H -[1,3]Dioxolo[4,5-e ]indol-8-yl)-N ,N -dimethylethan-1-amine
Other names
4,5-Methylenedioxy-N ,N -dimethyltryptamine
Identifiers
ChEMBL
ChemSpider
UNII
InChI=1S/C13H16N2O2/c1-15(2)6-5-9-7-14-10-3-4-11-13(12(9)10)17-8-16-11/h3-4,7,14H,5-6,8H2,1-2H3
Key: ZMKRWFZFMOKVCP-UHFFFAOYSA-N
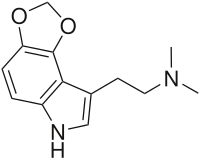
CN(C)CCC1=CNC2=C1C(OCO3)=C3C=C2
Properties
C 13 H 16 N 2 O 2
Molar mass
−1
Melting point
93–95 °C (199–203 °F; 366–368 K)[1]
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
Chemical compound
4,5-MDO-DMT , or 4,5-methylenedioxy-N ,N -dimethyltryptamine , is a lesser-known psychedelic drug . It is the 4,5-methylenedioxy analog of DMT . 4,5-MDO-DMT was first synthesized by Alexander Shulgin , though in his book TiHKAL psychoactive effects. 4,5-MDO-DMT has been the subject of limited subsequent testing; with behavioral disruption studies performed in male rats indicating that its hallucinogenic potency is less than that of 4,5-MDO-DiPT but greater than that of 5,6-MDO-DiPT .[1]
^ a b Kline, Toni B.; Benington, Frederick; Morin, Richard D.; Beaton, John M. (August 1982). "Structure-activity relationships in potentially hallucinogenic N,N-dialkyltryptamines substituted in the benzene moiety". Journal of Medicinal Chemistry . 25 (8): 908–913. doi :10.1021/jm00350a005 . PMID 7120280 .
1-Methylpsilocin 2,alpha-DMT 2-Me-DET 2-Methyl-5-HT 2,N,N-TMT 4,5-DHP-DMT 4,5-MDO-DMT 4,5-MDO-DiPT 4-AcO-DALT 4-AcO-DET 4-AcO-DMT 4-AcO-DiPT 4-AcO-EPT 4-AcO-NMT 4-AcO-MALT 4-AcO-MET 4-AcO-DPT 4-AcO-MiPT 4-F-5-MeO-DMT 4-HO-5-MeO-DMT 4-HO-DALT 4-HO-DBT 4-HO-DET 4-HO-DiPT 4-HO-DPT 4-HO-DSBT 4-HO-EPT 4-HO-MALT 4-HO-MET 4-HO-McPT 4-HO-McPeT 4-HO-MiPT 4-HO-MPMI 4-HO-MPT 4-HO-MsBT 4-HO-NMT 4-HO-PiPT 4-HO-pyr-T 4-HO-αMT 4-Me-αET 4-Me-αMT 4-MeO-DiPT 4-MeO-DMT 4-MeO-MiPT 4-PrO-DMT 5,6-MeO-MiPT 5,6-MDO-DiPT 5,6-MDO-DMT 5,6-MDO-MiPT 5,7-Dihydroxytryptamine 5-BT 5-Bromo-DMT 5-CT 5-Chloro-αMT 5-Chloro-DMT 5-Ethoxy-αMT 5-Ethoxy-DMT 5-Ethyl-DMT 5-Fluoro-AET 5-Fluoro-αMT 5-Fluoro-DET 5-Fluoro-DMT 5-Fluoro-EPT 5-Fluoro-MET 5-HO-αMT 5-HO-DiPT 5-HTP 5-iPrO-AMT 5-MeS-DMT 5-Methoxytryptamine 5-MeO-7,N,N-TMT 5-Methyl-αET 5-MeO-2-TMT 5-MeO-αET 5-MeO-αMT 5-MeO-DALT 5-MeO-DBT 5-MeO-DET 5-MeO-DiPT 5-MeO-DMT 5-MeO-DPT 5-MeO-EiPT 5-MeO-EPT 5-MeO-MALT 5-MeO-MET 5-MeO-MiPT 5-MeO-MPMI 5-MeO-NMT 5-MeO-pyr-T 5-MeO-NBpBrT 5-Methyl-DMT 5-(Nonyloxy)tryptamine 6-Fluoro-αMT 6-Fluoro-DMT 6-Hydroxymelatonin 6-MeO-THH 7-Chloro-AMT 7-Methyl-α-ethyltryptamine 7-Methyl-DMT Acetryptine Aeruginascin αET Alpha,N-DMT α,N,N-Trimethyltryptamine Alpha,N,O-TMS AL-37350A αMT Baeocystin BNC-210 Bufotenidine Bufotenin (5-HO-DMT) BW-723C86 Convolutindole A CP-132,484 DALT DBT Desformylflustrabromine DET DiPT DPT E-6801 E-6837 Ethocybin EiPT EMDT EPT FGIN-127 FGIN-143 Harmaline HIOC Ibogaine Idalopirdine Indorenate Iprocin Lespedamine Luzindole MET Methylbutyltryptamine MiPT MPT Miprocin Melatonin MPMI MS-245 NAS N-Ethyltryptamine N-Feruloylserotonin NMT DMT Norbaeocystin Normelatonin N-t-Butyltryptamine O-4310 Oxypertine Plakohypaphorine PiPT Psilocin (4-HO-DMT) Psilocybin (4-PO-DMT) Pyr-T Rizatriptan RU-28306 Serotonin ST-1936 Sumatriptan Tryptamine Tryptophan Yohimbine Yuremamine Zolmitriptan