Ruthenium tetroxide
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Ruthenium(VIII) oxide
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.039.815 | ||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| RuO4 | |||
| Molar mass | 165.07 g/mol | ||
| Appearance | yellow easily melting solid | ||
| Odor | pungent | ||
| Density | 3.29 g/cm3 | ||
| Boiling point | 129.6[1] °C (265.3 °F; 402.8 K) | ||
| 2% w/v at 20 °C | |||
| Solubility in other solvents | Soluble in Carbon tetrachloride Chloroform | ||
| Structure | |||
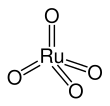
| tetrahedral | |||
| zero | |||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Safety data sheet (SDS) | external MSDS sheet | ||
| Related compounds | |||
Related compounds
|
Ruthenium dioxide Ruthenium trichloride | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Ruthenium tetroxide is the inorganic compound with the formula RuO4. It is a yellow volatile solid that melts near room temperature.[2] It has the odor of ozone.[3] Samples are typically black due to impurities. The analogous OsO4 is more widely used and better known. It is also the anhydride of hyperruthenic acid (H2RuO5). One of the few solvents in which RuO4 forms stable solutions is CCl4.[4]
Preparation
[edit]RuO4 is prepared by oxidation of ruthenium(III) chloride with NaIO4.[2] The reaction initially produces sodium diperiododihydroxoruthenate(VI), which then decomposes in acid solution to the tetroxide:[5]
- 8 Ru3+(aq) + 5 IO4−(aq) + 12 H2O(l) → 8 RuO4(s) + 5 I−(aq) + 24 H+(aq)[6]
Due to its challenging reactivity, RuO4 is always generated in situ and used in catalytic quantities, at least in organic reactions.[4]
Structure
[edit]RuO4 forms two crystal structures, one with cubic symmetry and another with monoclinic symmetry, isotypic to OsO4. The molecule adopts a tetrahedral geometry, with the Ru–O distances ranging from 169 to 170 pm.[7]
Uses
[edit]Isolation of ruthenium from ores
[edit]The main commercial value of RuO4 is as an intermediate in the production of ruthenium compounds and metal from ores. Like other platinum group metals (PGMs), ruthenium occurs at low concentrations and often mixed with other PGMs. Together with OsO4, it is separated from other PGMs by distillation of a chlorine-oxidized extract. Ruthenium is separated from OsO4 by reducing RuO4 with hydrochloric acid, a process that exploits the highly positive reduction potential for the [RuO4]0/- couple.[8][9]
Organic chemistry
[edit]RuO4 is of specialized value in organic chemistry because it oxidizes virtually any hydrocarbon. For example, it will oxidize adamantane to 1-adamantanol. Because it is such an aggressive oxidant, reaction conditions must be mild, generally room temperature. Although a strong oxidant, RuO4 oxidations do not perturb stereocenters that are not oxidized. Illustrative is the oxidation of the following diol to a carboxylic acid:
Oxidation of epoxy alcohols also occurs without degradation of the epoxide ring:
Under milder conditions, oxidative reaction yields aldehydes instead. RuO4 readily converts secondary alcohols into ketones. Although similar results can be achieved with other cheaper oxidants such as PCC- or DMSO-based oxidants, RuO4 is ideal when a very vigorous oxidant is needed, but mild conditions must be maintained. It is used in organic synthesis to oxidize internal alkynes to 1,2-diketones, and terminal alkynes along with primary alcohols to carboxylic acids. When used in this fashion, the ruthenium(VIII) oxide is used in catalytic amounts and regenerated by the addition of sodium periodate to ruthenium(III) chloride and a solvent mixture of acetonitrile, water and carbon tetrachloride. RuO4 readily cleaves double bonds to yield carbonyl products, in a manner similar to ozonolysis. OsO4, a more familiar oxidant that is structurally similar to RuO4, does not cleave double bonds, instead producing vicinal diol products. However, with short reaction times and carefully controlled conditions, RuO4 can also be used for dihydroxylation.[10]
Because RuO4 degrades the "double bonds" of arenes (especially electron-rich ones) by dihydroxylation and cleavage of the C-C bond in a way few other reagents can, it is useful as a "deprotection" reagent for carboxylic acids that are masked as aryl groups (typically phenyl or p-methoxyphenyl). Because the fragments formed are themselves readily oxidizable by RuO4, a substantial fraction of the arene carbon atoms undergo exhaustive oxidation to form carbon dioxide. Consequently, multiple equivalents of the terminal oxidant (often in excess of 10 equivalents per aryl ring) are required to achieve complete conversion to the carboxylic acid, limiting the practicality of the transformation.[11][12][13]
Although used as a direct oxidant, due to the relatively high cost of RuO4 it is also used catalytically with a cooxidant. For an oxidation of cyclic alcohols with RuO4 as a catalyst and bromate as oxidant under basic conditions, RuO4 is first activated by hydroxide, turning into the hyperruthenate anion:
- RuO4 + OH− → HRuO5−
The reaction proceeds via a glycolate complex.
Other uses
[edit]Ruthenium tetroxide is a potential staining agent. It is used to expose latent fingerprints by turning to the brown/black ruthenium dioxide when in contact with fatty oils or fats contained in sebaceous contaminants of the print.[14]
Gaseous release by nuclear accidents
[edit]Because of the very high volatility of ruthenium tetroxide (RuO
4) ruthenium radioactive isotopes with their relative short half-life are considered as the second most hazardous gaseous isotopes after iodine-131 in case of release by a nuclear accident.[15][3][16] The two most important radioactive isotopes of ruthenium are 103Ru and 106Ru. They have half-lives of 39.6 days and 373.6 days, respectively.[3]
References
[edit]- ^ Koda, Yoshio (1986). "Boiling Points and Ideal Solutions of Ruthenium and Osmium Tetraoxides". Journal of the Chemical Society, Chemical Communications. 1986 (17): 1347–1348. doi:10.1039/C39860001347.
- ^ a b H. L. Grube (1963). "Ruthenium (VIII) Oxide". In G. Brauer (ed.). Handbook of Preparative Inorganic Chemistry, 2nd Ed. Vol. 1. NY: Academic Press. pp. 1599–1600.
- ^ a b c Backman, U., Lipponen, M., Auvinen, A., Jokiniemi, J., & Zilliacus, R. (2004). Ruthenium behaviour in severe nuclear accident conditions. Final report (No. NKS–100). Nordisk Kernesikkerhedsforskning.
- ^ a b Martín, V. S.; Palazón, J. M.; Rodríguez, C. M.; Nevill, C. R. (2006). "Ruthenium(VIII) Oxide". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rr009.pub2. ISBN 978-0471936237.
- ^ Mercer, E. E.; Meyer, S. M. (1972) [1 July 1971]. "A periodate complex of ruthenium(VI)". J. Inorg. Nucl. Chem. 34 (2). Great Britain: Pergamon: 777–778. doi:10.1016/0022-1902(72)80466-4.
- ^ Carlsen, Per H. J.; Katsuki, Tsutomu; Martin, Victor S.; Sharpless, K. Barry (September 1981). "A greatly improved procedure for ruthenium tetroxide catalyzed oxidations of organic compounds". The Journal of Organic Chemistry. 46 (19): 3936–3938. doi:10.1021/jo00332a045. ISSN 0022-3263.
- ^ Pley, M.; Wickleder, M. S. (2005). "Two Crystalline Modifications of RuO4". Journal of Solid State Chemistry. 178 (10): 3206–3209. Bibcode:2005JSSCh.178.3206P. doi:10.1016/j.jssc.2005.07.021.
- ^ Bernardis, Francesco L.; Grant, Richard A.; Sherrington, David C. (2005). "A review of methods of separation of the platinum-group metals through their chloro-complexes". Reactive and Functional Polymers. 65 (3): 205–217. doi:10.1016/j.reactfunctpolym.2005.05.011.
- ^ Swain, P.; Mallika, C.; Srinivasan, R.; Mudali, U. K.; Natarajan, R. (2013). "Separation and recovery of ruthenium: a review". Journal of Radioanalytical and Nuclear Chemistry. 298 (2): 781–796. doi:10.1007/s10967-013-2536-5. S2CID 95804621.
- ^ Plietker, Bernd (2005). "Selectivity versus reactivity - recent advances in RuO4-catalyzed oxidations". Synthesis. 5 (15): 2453–2472. doi:10.1055/s-2005-872172.
- ^ Nunez, M. Teresa; Martin, Victor S. (March 1990). "Efficient oxidation of phenyl groups to carboxylic acids with ruthenium tetraoxide. A simple synthesis of (R)-.gamma.-caprolactone, the pheromone of Trogoderma granarium". The Journal of Organic Chemistry. 55 (6): 1928–1932. doi:10.1021/jo00293a044. ISSN 0022-3263.
- ^ Nasr, Khaled; Pannier, Nadine; Frangioni, John V.; Maison, Wolfgang (February 2008). "Rigid Multivalent Scaffolds Based on Adamantane". The Journal of Organic Chemistry. 73 (3): 1056–1060. doi:10.1021/jo702310g. ISSN 0022-3263. PMC 2505186. PMID 18179237.
- ^ Mander, Lewis N.; Williams, Craig M. (2003-02-17). "Oxidative degradation of benzene rings". Tetrahedron. 59 (8): 1105–1136. doi:10.1016/S0040-4020(02)01492-8. ISSN 0040-4020.
- ^ Mashiko, K.; Miyamoto, T. (1998). "Latent Fingerprint Processing by the Ruthenium Tetroxide Method". Journal of Forensic Identification. 48 (3): 279–290. doi:10.3408/jasti.2.21.
- ^ Ronneau, C.; Cara, J.; Rimski-Korsakov, A. (1995). "Oxidation-enhanced emission of ruthenium from nuclear fuel". Journal of Environmental Radioactivity. 26: 63–70. doi:10.1016/0265-931X(95)91633-F.
- ^ Beuzet, Emilie; Lamy, Jean-Sylvestre; Perron, Hadrien; Simoni, Eric; Ducros, Gérard (2012). "Ruthenium release modelling in air and steam atmospheres under severe accident conditions using the MAAP4 code". Nuclear Engineering and Design. 246: 157–162. doi:10.1016/j.nucengdes.2011.08.025.
Further reading
[edit]- Cotton, S.A. (1997). Chemistry of Precious Metals. London: Chapman and Hall. ISBN 978-0-7514-0413-5.
- Farmer, V.; Welton, T. (2002). "The oxidation of alcohols in substituted imidazolium ionic liquids using ruthenium catalysts". Green Chemistry. 4 (2): 97. doi:10.1039/B109851A.
- Singh, B.; Srivastava, S. (1991). "Kinetics and mechanism of ruthenium tetroxide catalysed oxidation of cyclic alcohols by bromate in a base". Transition Metal Chemistry. 16 (4): 466. doi:10.1007/BF01129466. S2CID 95711945.
- Courtney, J.L.; Swansbor, K.F. (1972). "Ruthenium tetroxide oxidation". Reviews of Pure and Applied Chemistry. 22: 47.