Carbon tetraiodide
| |||
 Carbon tetraiodide crystals (left)
Solution in Et2O (right) | |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Tetraiodomethane[1] | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 1733108 | |||
| ChemSpider | |||
| ECHA InfoCard | 100.007.335 | ||
| EC Number |
| ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| CI4 | |||
| Molar mass | 519.629 g·mol−1 | ||
| Appearance | Dark violet crystals | ||
| Density | 4.32 g mL−1 | ||
| -136·10−6 cm3/mol | |||
| Structure | |||
| Tetragonal | |||
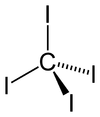
| Tetrahedral | |||
| 0 D | |||
| Thermochemistry | |||
Heat capacity (C)
|
0.500 J K−1 g−1 | ||
Std enthalpy of
formation (ΔfH⦵298) |
384.0–400.4 kJ mol−1 | ||
Std enthalpy of
combustion (ΔcH⦵298) |
−794.4–−778.4 kJ mol−1 | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
toxic | ||
| GHS labelling: | |||

| |||
| Warning | |||
| H315, H319, H335 | |||
| P261, P305+P351+P338 | |||
| Related compounds | |||
Other anions
|
Carbon tetrafluoride Carbon tetrachloride Carbon tetrabromide | ||
Other cations
|
Silicon tetraiodide Germanium tetraiodide Tin(IV) iodide | ||
Related alkanes
|
|||
Related compounds
|
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Carbon tetraiodide is a tetrahalomethane with the molecular formula . Being bright red, it is a relatively rare example of a highly colored methane derivative. It is only 2.3% by weight carbon, although other methane derivatives are known with still less carbon.
Structure
[edit]The tetrahedral molecule features C-I distances of 2.12 ± 0.02 Å.[2] The molecule is slightly crowded with short contacts between iodine atoms of 3.459 ± 0.03 Å, and possibly for this reason, it is thermally and photochemically unstable.
Carbon tetraiodide crystallizes in tetragonal crystal structure (a 6.409, c 9.558 (.10−1 nm)).[3]
It has zero dipole moment due to its symmetrically substituted tetrahedral geometry.
Properties, synthesis, uses
[edit]Carbon tetraiodide is slightly reactive towards water, giving iodoform and I2. It is soluble in nonpolar organic solvents. It decomposes thermally and photochemically to tetraiodoethylene, C2I4. Its synthesis entails AlCl3-catalyzed halide exchange, which is conducted at room temperature:[4]
The product crystallizes from the reaction solution.
Carbon tetraiodide is used as an iodination reagent, often upon reaction with bases.[5] Ketones are converted to 1,1-diiodoalkenes upon treatment with triphenylphosphine (PPh3) and carbon tetraiodide. Alcohols are converted in and to iodide, by a mechanism similar to the Appel reaction. In an Appel reaction, carbon tetrachloride is used to generate alkyl chlorides from alcohols.
Safety considerations
[edit]Manufacturers recommend that carbon tetraiodide be stored near 0 °C (32 °F). As a ready source of iodine, it is an irritant. Its LD50 on rats is 18 mg/kg. In general, perhalogenated organic compounds should be considered toxic, with the narrow exception of small perfluoroalkanes (essentially inert due to the strength of the C-F bond).
References
[edit]- ^ "Tetraiodomethane - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 27 March 2005. Identification and Related Records. Retrieved 29 February 2012.
- ^ Finbak, Chr.; Hassel, O. (1937). "Kristallstruktur und Molekülbau von CI4 und CBr4". Zeitschrift für Physikalische Chemie. B36: 301–308. doi:10.1515/zpch-1937-3621. S2CID 99718985.
- ^ Pohl, S. (1982). "Die Kristallstruktur von CI4". Zeitschrift für Kristallographie. 159 (1–4): 211–216. doi:10.1524/zkri.1982.159.14.211. S2CID 102246815.
- ^ McArthur, R. E.; Simons, J. H. (1950). "Carbon Tetraiodide". Inorganic Syntheses. Inorganic Syntheses. Vol. III. pp. 37–39. doi:10.1002/9780470132340.ch8. ISBN 9780470132340.
- ^ P. R. Schreiner, A. A. Fokin (2005). "Carbon Tetraiodide". In L. Paquette (ed.). Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons, Ltd.
Further reading
[edit]- Sorros H., Hinkam J. B. (1945). "The Redistribution Reaction. XI. Application to the Preparation of Carbon Tetraiodide and Related Halides". Journal of the American Chemical Society. 67 (10): 1643. doi:10.1021/ja01226a004.




