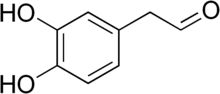
3,4-Dihydroxyphenylacetaldehyde
| |
| Names | |
|---|---|
| Preferred IUPAC name
(3,4-Dihydroxyphenyl)acetaldehyde | |
| Other names
2-(3,4-Dihydroxyphenyl)acetaldehyde[1]
Dopaldehyde | |
| Identifiers | |
3D model (JSmol)
|
|
| 3DMet | |
| Abbreviations | DOPAL |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.237.172 |
| KEGG | |
| MeSH | 3,4-dihydroxyphenylacetaldehyde |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8H8O3 | |
| Molar mass | 152.149 g·mol−1 |
| Density | 1.306 g/mL |
| Boiling point | 351 °C (664 °F; 624 K) |
| Related compounds | |
Related 2-phenyl aldehydes
|
Phenylacetaldehyde |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
3,4-Dihydroxyphenylacetaldehyde (DOPAL) is an important metabolite of the major brain neurotransmitter dopamine. All of the enzymatic metabolism of dopamine in neurons passes through DOPAL. According to the "catecholaldehyde hypothesis," DOPAL plays a role in the pathogenesis of Parkinson's disease.[2] DOPAL is detoxified mainly by aldehyde dehydrogenase. DOPAL is a metabolite of dopamine by monoamine oxidase activity, or MAO, in differentiated neuronal cells of the PC12 line.[3] Physiological concentrations of DOPAL in isolated mitochondria were highly potent in inducing a pathway associated with programmed cell death (or apoptosis), permeability transition. This suggests the cytotoxity of DOPAL and its role in the progression of Parkinson's disease, which has long been associated with mitochondrial abnormalities and neurotoxicity by way of dopaminergic compounds, while reducing the emphasis on other dopamine derivatives and metabolites.[3]
References
[edit]- ^ "3,4-dihydroxyphenylacetaldehyde - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 24 June 2005. Identification and Related Records. Retrieved 13 October 2011.
- ^ Goldstein, David S.; Sullivan, Patti; Holmes, Courtney; Miller, Gary W.; Alter, Shawn; Strong, Randy; Mash, Deborah C.; Kopin, Irwin J.; Sharabi, Yehonatan (2013). "Determinants of buildup of the toxic dopamine metabolite <SCP>DOPAL</SCP> in Parkinson's disease". Journal of Neurochemistry. 126 (5): 591–603. doi:10.1111/jnc.12345. PMC 4096629. PMID 23786406.
- ^ a b Kristal, B.; Conway, A. D.; Brown, A. M.; Jain, J. C.; Ulluci, P. A.; Li, S. W.; Burke, W. J. (2001). "Selective dopaminergic vulnerability: 3,4-dihydroxyphenylacetaldehyde targets mitochondria". Free Radical Biology and Medicine. 30 (8): 924–931. doi:10.1016/s0891-5849(01)00484-1. PMID 11295535.
