Butene
This article needs additional citations for verification. (March 2024) |
Butene, also known as butylene, is an alkene with the formula C4H8. The word butene may refer to any of the individual compounds. They are colourless gases that are present in crude oil as a minor constituent in quantities that are too small for viable extraction. Butene is therefore obtained by catalytic cracking of long-chain hydrocarbons left during refining of crude oil. Cracking produces a mixture of products, and the butene is extracted from this by fractional distillation.[1]
Butene can be used as the monomer for polybutene, but this polymer is more expensive than alternatives with shorter carbon chains such as polypropylene. Polybutene is therefore used in more specialized applications. Butenes are more commonly used to make copolymer (mixed with another monomer such as ethylene).
Butenes are major constituents of raffinates, the C4 fractions in oil processing. The raffinates containing butadiene are considered carcinogenic and mutagenic.[2] They can be used as feedstocks for further processing, or used as industrial fuel. Their mixing into LPG for nonindustrial uses sometimes occurs but is generally prohibited.[3]
Isomers
[edit]Among the molecules which have the chemical formula C4H8 four isomers are alkenes. All four of these hydrocarbons have four carbon atoms and one double bond in their molecules, but have different chemical structures. The IUPAC and common names, respectively, of these chemical compounds are:
| Common name(s) | IUPAC name | Structure | Skeletal formula | 3D model |
|---|---|---|---|---|
| 1-butene, α-butylene | But-1-ene | 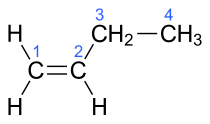
|

| |
| cis-2-butene, cis-β-butylene | (2Z)-but-2-ene | 
|

|

|
| trans-2-butene, trans-β-butylene | (2E)-but-2-ene | 
|

|

|
| isobutene, isobutylene | 2-methylprop-1-ene | 
|

|

|
In the chemical structures above, the small blue numbers in the structure images are the numbering of the atoms in the main backbone chain of the molecules. Other organic compounds have the formula C4H8, namely cyclobutane and methylcyclopropane, but are not alkenes and do not fall under the name butene. There are also cyclic alkenes with four carbon atoms overall such as cyclobutene and two isomers of methylcyclopropene, but they do not have the formula C4H8 and are not discussed here.
Properties
[edit]All four of these isomers are gases at room temperature and pressure, but can be liquefied by lowering the temperature or raising the pressure on them, in a manner similar to pressurised butane. These gases are colourless, do have distinct odours, and are highly flammable. Although not naturally present in petroleum in high percentages, they can be produced from petrochemicals or by catalytic cracking of petroleum. Although they are stable compounds, the carbon-carbon double bonds make them more reactive than similar alkanes, which are more inert compounds in various ways.
Because of the double bonds, these 4-carbon alkenes can act as monomers in the formation of polymers, as well as having other uses as petrochemical intermediates. They are used in the production of synthetic rubber. But-1-ene is a linear or normal alpha-olefin and isobutylene is a branched alpha-olefin. In a rather low percentage, but-1-ene is used as one of the comonomers, along with other alpha-olefins, in the production of high-density polyethylene and linear low-density polyethylene. Butyl rubber is made by cationic polymerisation of isobutylene with about 2 - 7% isoprene. Isobutylene is also used for the production of methyl tert-butyl ether (MTBE) and isooctane, both of which improve the combustion of gasoline.
Occurrence and Production
[edit]Butenes are naturally present in crude oil, albeit in small quantities that make direct extraction economically unfeasible. Instead, these valuable compounds are primarily obtained through a process called catalytic cracking. This industrial method involves breaking down longer hydrocarbon chains, which are less valuable byproducts of crude oil refining, into shorter, more useful molecules like butene.
The catalytic cracking process yields a mixture of various hydrocarbons. To isolate butene from this complex mixture, petrochemical engineers employ fractional distillation. This separation technique exploits the different boiling points of the compounds in the mixture, allowing for the efficient extraction of butene.
Industrial Applications
[edit]Polymer Production
[edit]Butene serves as a monomer in the production of polybutene, a type of polymer. However, due to its higher cost compared to alternatives like polypropylene (which uses propene as a monomer), polybutene finds its niche in more specialized applications where its unique properties justify the additional expense.
More commonly, butenes are utilized in the production of copolymers. In this process, butene is combined with another monomer, often ethylene, to create materials with properties that can be fine-tuned for specific applications.
Refinery Products
[edit]Butenes are major components of raffinates, which are C4 fractions produced during oil processing. These raffinates, particularly those containing butadiene, are considered hazardous due to their carcinogenic and mutagenic properties. Despite these risks, raffinates serve as valuable feedstocks for further processing in the petrochemical industry.
In some cases, raffinates are used as industrial fuel. However, their incorporation into liquefied petroleum gas (LPG) for non-industrial uses is generally prohibited due to safety concerns and regulatory restrictions.
Environmental and Safety Considerations
[edit]While butenes are valuable industrial chemicals, their production and use come with environmental and safety challenges. The raffinates containing butadiene, a common byproduct in butene production, are known carcinogens and mutagens. This necessitates stringent safety measures in industrial settings where these compounds are handled.
Moreover, the high flammability of butenes poses fire and explosion risks, requiring careful storage and handling procedures. The potential environmental impact of butene production and use, particularly in the context of fossil fuel dependence and plastic pollution, is an ongoing area of concern and research in the chemical industry.
Conclusion
[edit]Butene, with its four isomers, plays a crucial role in the petrochemical industry. From its production through catalytic cracking to its diverse applications in polymer production and fuel additives, butene exemplifies the complex interplay of chemistry, engineering, and industrial processes that characterize modern petrochemical operations. As the industry continues to evolve, driven by environmental concerns and the search for more sustainable practices, the role of butene and similar compounds will likely continue to be a subject of innovation and adaptation.
See also
[edit]References
[edit]- ^ Geilen, Frank M.A.; Stochniol, Guido; Peitz, Stephan; Schulte-Koerne, Ekkehard (2014). "Butenes". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a04_483.pub3. ISBN 978-3527306732.
- ^ Safety Data Sheet pcs.com.sg April 2017
- ^ "Karcinogenní plyn na českém trhu není, sdělila asociace. Inspekce zmírnila své zjištění | Náš REGION".
External links
[edit]
