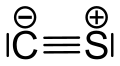Carbon monosulfide
Appearance
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
carbon monosulfide
| |||
| Other names
carbon(II) sulfide, thiocarbonyl, sulfidocarbon, methanidylidynesulfanium
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 1697516, 1918616 | |||
| ChEBI | |||
| ChemSpider | |||
| 648 | |||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| CS | |||
| Molar mass | 44.07 g·mol−1 | ||
| Appearance | reddish crystalline powder | ||
| insoluble | |||
| Related compounds | |||
Other anions
|
Carbon monoxide | ||
Other cations
|
Silicon monosulfide Germanium monosulfide Tin(II) sulfide Lead(II) sulfide | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Carbon monosulfide is a chemical compound with the formula CS. This diatomic molecule is the sulfur analogue of carbon monoxide, and is unstable as a solid or a liquid, but it has been observed as a gas both in the laboratory and in the interstellar medium.[1] The molecule resembles carbon monoxide with a triple bond between carbon and sulfur. The molecule is not intrinsically unstable, but it tends to polymerize. This tendency reflects the greater stability of C–S single bonds.
Polymers with the formula (CS)n have been reported,[2] and the formal dimer is ethenedithione. Also, CS has been observed as a ligand in some transition metal complexes.
References
[edit]- ^ Wilson, R. W.; Penzias, A. A.; Wannier, P. G.; Linke, R. A. (1976). "Isotopic abundances in interstellar carbon monosulfide". Astrophysical Journal. 204 (pt 2): L135–L137. Bibcode:1976ApJ...204L.135W. doi:10.1086/182072.
- ^ Chou, J.-H.; Rauchfuss, T. B. (1997). "Solvatothermal Routes to Poly(Carbon Monosulfide)s Using Kinetically Stabilized Precursors" (PDF). Journal of the American Chemical Society. 119 (19): 4537–4538. doi:10.1021/ja970042w.