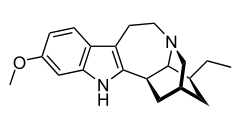
Tabernanthine
Appearance
 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C20H26N2O |
| Molar mass | 310.441 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Tabernanthine is an alkaloid found in Tabernanthe iboga.[1]
It has been used in laboratory experiments to study how addiction affects the brain.[2]
Tabernanthine persistently reduced the self-administration of cocaine and morphine in rats.[3]
Pharmacology
[edit]It is kappa opioid agonist (Ki = 0.15 μM) and NMDA receptor (Ki = 10.5 μM) antagonist.[4][5] Compared to ibogaine, it binds weakly to σ1 and σ2 receptor.[5]
See also
[edit]References
[edit]- ^ Bartlett MF, Dickel DF, Taylor WI (1958). "The Alkaloids of Tabernanthe iboga. Part IV.1 The Structures of Ibogamine, Ibogaine, Tabernanthine and Voacangine". Journal of the American Chemical Society. 80: 126–136. doi:10.1021/ja01534a036.
- ^ Levi MS, Borne RF (October 2002). "A review of chemical agents in the pharmacotherapy of addiction". Current Medicinal Chemistry. 9 (20): 1807–1818. doi:10.2174/0929867023368980. PMID 12369879.
- ^ Glick SD, Kuehne ME, Raucci J, Wilson TE, Larson D, Keller RW, Carlson JN (September 1994). "Effects of iboga alkaloids on morphine and cocaine self-administration in rats: relationship to tremorigenic effects and to effects on dopamine release in nucleus accumbens and striatum". Brain Research. 657 (1–2): 14–22. doi:10.1016/0006-8993(94)90948-2. PMID 7820611. S2CID 1940631.
- ^ Deecher DC, Teitler M, Soderlund DM, Bornmann WG, Kuehne ME, Glick SD (February 1992). "Mechanisms of action of ibogaine and harmaline congeners based on radioligand binding studies". Brain Research. 571 (2): 242–247. doi:10.1016/0006-8993(92)90661-r. PMID 1377086. S2CID 17159661.
- ^ a b Wiart C (16 December 2013). Lead Compounds from Medicinal Plants for the Treatment of Neurodegenerative Diseases. Academic Press. pp. 67–69, 73. ISBN 978-0-12-398383-1.
