Политиазил

| |

| |

| |
| Имена | |
|---|---|
| Другие имена
политиазил
поли (нитрид серы) | |
| Идентификаторы | |
| Chemspider |
|
| Характеристики | |
| (SN)x | |
| Appearance | Golden or bronze-coloured crystalline solid with metallic lustre[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Политиазил ( полимерный серы нитрид ), (SN) X , представляет собой электрически проводящий , золотой или бронзовый полимер с металлическим блеском . первый проводящий неорганический полимер Это был [ 1 ] [ 2 ] и было также обнаружено, что он является сверхпроводником при очень низких температурах (ниже 0,26 К). [ 3 ] [ 4 ] Это волокнистое твердое вещество, описанное как «блестящий золотистый на лицах и темно-сине-черный», в зависимости от ориентации образца. Это воздушное стабильное и нерастворимое во всех растворителях. [ 5 ]
История
[ редактировать ]Сначала сообщили об этом соединении еще в 1910 году ФП Бертом, который получил его путем нагрева тетранитрида тетрасульфура в вакууме над серебряной шерстью. [ 6 ]
Соединение было первым соединением только с неметаллическими элементами, в которых сверхпроводимость можно продемонстрировать . Тем не менее, относительно низкая температура перехода при 0,3 К делает практическое применение маловероятным. [ 7 ] [ 8 ]
Properties
[edit]Polythiazyl is a metallic-golden and shiny, crystalline but fibrous material.[8] The polymer is mostly inert to oxygen and water, but decomposes in air to a grey powder.[9][10] At temperatures above 240 °C explosive decomposition can occur.[11] The compound also explodes on impact.[10] Explosion generally proceeds via decomposition to the elements.
Polythiazyl shows an anisotropic electrical conductivity. Along the fibres or SN chains, the bond is electrically conductive, perpendicular to it acts as an insulator. The one-dimensional conductivity is based on the bonding conditions in the S-N chain, where each sulfur atom provides two π electrons and each nitrogen atom provides one π electron to form two-center 3π electron bonding units.[8]
Two polymorphic crystal forms were observed in the compound. The monoclinic form I obtained from the synthesis can be converted into an orthorhombic form II by mechanical treatment such as grinding.[12]
Structure and bonding
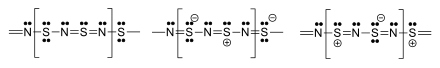
[edit]The material is a polymer, containing trivalent nitrogen, and divalent and tetravalent sulfur. The S and N atoms on adjacent chains align.[2][13][14] Several resonance structures can be written.[15]
The structure of the crystalline compound was resolved by X-ray diffraction. This showed alternating S–N bond lengths of 159 pm and 163 pm and S–N–S bond angles of 120 ° and N–S–N bond angles of 106 °.[16][17][9][8]
Synthesis
[edit]Polythiazyl is synthesized by the polymerization of the cyclic formal dimer disulfur dinitride (S2N2), which is in turn synthesized from the formal tetramer tetrasulfur tetranitride (S4N4),[2] in the presence of hot silver wool.[2][1][18]
The reaction begins when silver abstracts sulfur from S4N4 to produce a Ag2S catalyst; the resulting gaseous S2N2 is then isolated through sublimation onto a cold surface:
- S4N4 + 8 Ag → 4 Ag2S + 2 N2
- S4N4 (low-pressure gas at 250-300 °C; Ag2S catalyst) → 2 S2N2 (gas) → 2 S2N2 (stable solid at 77 K)
When warmed to room temperature, the additional heat induces spontaneous polymerization:
- S2N2 (0 °C) → (SN)x
Uses
[edit]Due to its electrical conductivity, polythiazyl is used in LEDs, transistors, battery cathodes, and solar cells.[18]
Literature
[edit]King, R.S.P.: Novel chemistry and applications of polythiazyl, Doctoral Thesis Loughborough University 2009, pdf-Download
References
[edit]- ^ Jump up to: a b c Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. pp. 725–727. ISBN 978-0-08-037941-8.
- ^ Jump up to: a b c d Goehring, Margot; Voigt, Dietrich (1953). "Über die Schwefelnitride (SN)2 und (SN)x". Die Naturwissenschaften (in German). 40 (18): 482. Bibcode:1953NW.....40..482G. doi:10.1007/BF00628990. ISSN 0028-1042. S2CID 8181710.
- ^ Labes, M. M.; Love, P.; Nichols, L. F. (1979). "Polysulfur Nitride - a Metallic, Superconducting Polymer". Chemical Reviews. 79 (1): 1–15. doi:10.1021/cr60317a002.
- ^ Harry R. Allcock (20 September 2011). Introduction to Materials Chemistry. John Wiley & Sons. p. 131. ISBN 978-1-118-21098-7. Retrieved 29 June 2012.
- ^ A. G. MacDiarmid; C. M. Mikulsk; A. J. Heeger; A. F. Garito (1983). "Polymeric Sulfur Nitride (Polythiazyl), (SN) X". Polymeric Sulfur Nitride (Polythiazyl), (SN)x. Inorganic Syntheses. Vol. 22. pp. 143–149. doi:10.1002/9780470132531.ch31. ISBN 9780470132531.
- ^ Burt, Frank Playfair (1910). "XCIX.—A new sulphide of nitrogen". J. Chem. Soc., Trans. 97: 1171–1174. doi:10.1039/CT9109701171. ISSN 0368-1645.
- ^ Labes, M.M.; Love, P.; Nichols, L.F.: Polysulfur nitride - a metallic, superconducting polymer in Chem. Rev. 79 (1979) 1–15, doi:10.1021/cr60317a002.
- ^ Jump up to: a b c d Alsfasser, R.; Janiak, C.; Klapötke, T.M.; Meyer, H.-J.: Moderne Anorganische Chemie, Herausgeber Riedel, E., 3. Auflage 2007, Walter de Gruyter GmbH & Co. KG, Berlin/Boston, ISBN 978-3-11-019060-1, S. 129–132, (retrieved via De Gruyter Online).
- ^ Jump up to: a b MacDiarmid, A.G.; Mikulski, C.M.; Saran, M.S.; Russo, P.J.; Cohen, M.J.; Bright, A.A.; Garito, A.F.; Heeger, A.J.: Synthesis and Selected Properties of Polymeric Sulfur Nitride, (Polythiazyl), (SN)x in Advances in Chemistry 150 (2009) 63–72, doi:10.1021/ba-1976-0150.ch006.
- ^ Jump up to: a b Entry on Schwefel-Stickstoff-Verbindungen. at: Römpp Online. Georg Thieme Verlag, retrieved 2 March 2017.
- ^ Wiberg, E.; Wiberg, N.; Holleman, A.F.: Anorganische Chemie, 103. Auflage, 2017 Walter de Gruyter GmbH & Co. KG, Berlin/Boston, ISBN 978-3-11-026932-1, S. 681, (retrieved via De Gruyter Online).
- ^ Baughman, R.H.; Apgar, P.A.; Chance, R.R.; MacDiarmid, A.G.; Garito, A.F.: A New Phase of (SN)x in J. Chem. Soc. Chem. Comm. 1977, 49–50, doi:10.1039/C39770000049.
- ^ Goehring, Margot (1956). "Sulphur nitride and its derivatives". Quarterly Reviews, Chemical Society. 10 (4): 437. doi:10.1039/qr9561000437. ISSN 0009-2681.
- ^ Cohen, M.J .; Garito, A. F.; Heeger, A. J.; MacDiarmid, A. G.; Mikulski, C. M.; Saran, M. S.; Kleppinger, J. (1976). "Solid state polymerization of S2N2 to (SN)x". Journal of the American Chemical Society. 98: 3844–3848. doi:10.1021/ja00429a018.
- ^ Okada, M.; Tanaka, K.; Takata, A.; Yamabe, T. (1993). "Examination of Electronic Phase of the Hartree-Fock Solution of an Isolated Polythiazyl Chain". Synthetic Metals. 59 (2): 223–230. doi:10.1016/0379-6779(93)91029-2.
- ^ Boudeulle, M.: in Cryst. Struct. Comm. 4 (1975) 9–13.
- ^ MacDiarmid, A.G.; Mikulski, C.M.; Russo, P.J.; Saran, M.S.; Garito, A.F.; Heeger, A.J.: Synthesis and structure of the polymeric metal, (SN)x, and its precursor, S2N2 in J. Chem. Soc. Chem. Comm. 1975, 476–477, doi:10.1039/C39750000476.
- ^ Jump up to: a b Ronald D. Archer (26 February 2001). Inorganic and Organometallic Polymers. John Wiley & Sons. p. 213. ISBN 978-0-471-24187-4. Retrieved 29 June 2012.