Основные белки мочи

Основные белки мочи ( MUP ), также известные как α 2 U-Глобулины , представляют собой подсемейство белков, обнаруженных в изобилии в моче и других секретах многих животных. Mups предоставляют небольшой диапазон идентифицирующей информации о донорском животном, когда обнаруживается вороназальным органом приемного животного. Они принадлежат к более крупному семейству белков, известных как липокалины . Mups кодируются кластером генов , расположенных рядом друг с другом на одном участке ДНК, который сильно варьируется по количеству между видами: от по меньшей мере от 21 функциональных генов у мышей до человека. Белки MUP образуют характерную форму перчатки , охватывая лиганд -связывающий карман, который вмещает определенные небольшие органические химические вещества.
Белки мочи впервые были зарегистрированы у грызунов в 1932 году, во время исследований Томаса Аддиса по причине протеинурии . Они являются мощными человеческими аллергенами и в значительной степени ответственны за ряд аллергии на животных , в том числе для кошек, лошадей и грызунов. Их эндогенная функция внутри животного неизвестна, но может включать регулирование расходов на энергию. Однако в качестве секретируемых белков они играют множественную роль в химической связи между животными, функционируя как переносчики -феромоны и стабилизаторы у грызунов и свиней. Mups также могут выступать в качестве белковых феромонов. Было продемонстрировано, что они способствуют агрессии у самцов мышей, а один специфический белок MUP, обнаруженный у мочи мыши, сексуально привлекателен для самки мышей. Mups также могут функционировать как сигналы между различными видами : мыши демонстрируют инстинктивный ответ страха при обнаружении MUP, полученных из хищников, таких как кошки и крысы.
Discovery
[edit]
Humans in good health excrete urine that is largely free of protein. Therefore, since 1827 physicians and scientists have been interested in proteinuria, the excess of protein in human urine, as an indicator of kidney disease.[notes 1][2] To better understand the etiology of proteinuria, some scientists attempted to study the phenomenon in laboratory animals.[3] Between 1932 and 1933 a number of scientists, including Thomas Addis, independently reported the surprising finding that some healthy rodents have protein in their urine.[4][5][6] However, it was not until the 1960s that the major urinary proteins of mice and rats were first described in detail.[7][8] It was found that the proteins are primarily made in the liver of males and secreted through the kidneys into the urine in large quantities (milligrams per day).[7][8][9]
Since they were named, the proteins have been found to be differentially expressed in other glands that secrete products directly into the external environment. These include lacrimal, parotid, submaxillary, sublingual, preputial and mammary glands.[10][11][12] In some species, such as cats and pigs, Mups appear not to be expressed in urine at all and are mainly found in saliva.[13][14] Sometimes the term urinary Mups (uMups) is used to distinguish those Mups expressed in urine from those in other tissues.[15]
Mup genes
[edit]Between 1979 and 1981, it was estimated that Mups are encoded by a gene family of between 15 and 35 genes and pseudogenes in the mouse and by an estimated 20 genes in the rat.[16][17][18] In 2008 a more precise number of Mup genes in a range of species was determined by analyzing the DNA sequence of whole genomes.[1][19]
Rodents
[edit]
The mouse reference genome has at least 21 distinct Mup genes (with open reading frames) and a further 21 Mup pseudogenes (with reading frames disrupted by a nonsense mutation or an incomplete gene duplication). They are all clustered together, arrayed side by side across 1.92 megabases of DNA on chromosome 4. The 21 functional genes have been divided into two sub-classes based on position and sequence similarity: 6 peripheral Class A Mups and 15 central Class B Mups.[1][20] The central Class B Mup gene cluster formed through a number of sequential duplications from one of the Class A Mups. As all the Class B genes are almost identical to each other, researchers have concluded that these duplications occurred very recently in mouse evolution. Indeed, the repetitive structure of these central Mup genes means they are likely to be unstable and may vary in number among wild mice.[20] The Class A Mups are more different from each other and are therefore likely to be more stable, older genes, but what, if any, functional differences the classes have are unknown.[1] The similarity between the genes makes the region difficult to study using current DNA sequencing technology. Consequently, the Mup gene cluster is one of the few parts of the mouse whole genome sequence with gaps remaining, and further genes may remain undiscovered.[1][20]
Rat urine also contains homologous urinary proteins; although they were originally given a different name, α2u-globulins,[8][9] they have since become known as rat Mups.[21][22] Rats have 9 distinct Mup genes and a further 13 pseudogenes clustered together across 1.1 megabases of DNA on chromosome 5. Like in mice, the cluster formed by multiple duplications. However, this occurred independently of the duplications in mice, meaning that both rodent species expanded their Mup gene families separately, but in parallel.[1][23]
Nonrodents
[edit]Most other mammals studied, including the pig, cow, cat, dog, bushbaby, macaque, chimpanzee and orangutan, have a single Mup gene. Some, however, have an expanded number: horses have three Mup genes, and gray mouse lemurs have at least two. Insects, fish, amphibia, birds and marsupials appear to have disrupted synteny at the chromosomal position of the Mup gene cluster, suggesting the gene family may be specific to placental mammals.[1] Humans are the only placental mammals found not to have any active Mup genes; instead, they have a single Mup pseudogene containing a mutation that causes missplicing, rendering it dysfunctional.[1]
Function
[edit]Transport proteins
[edit]
Mups are members of a large family of low-molecular weight (~19 kDa) proteins known as lipocalins.[25] They have a characteristic structure of eight beta sheets arranged in an anti-parallel beta barrel open on one face, with alpha helices at both ends.[25] Consequently, they form a characteristic glove shape, encompassing a cup-like pocket that binds small organic chemicals with high affinity.[1][26] A number of these ligands bind to mouse Mups, including 2-sec-butyl-4,5-dihydrothiazole (abbreviated as SBT or DHT), 6-hydroxy-6-methyl-3-heptanone (HMH) and 2,3 dihydro-exo-brevicomin (DHB).[27][28][29] These are all urine-specific chemicals that have been shown to act as pheromones—molecular signals excreted by one individual that trigger an innate behavioural response in another member of the same species.[27][30] Mouse Mups have also been shown to function as pheromone stabilizers, providing a slow release mechanism that extends the potency of volatile pheromones in male urine scent marks.[31] Given the diversity of Mups in rodents, it was originally thought that different Mups may have differently shaped binding pockets and therefore bind different pheromones. However, detailed studies found that most variable sites are located on the surface of the proteins and appear to have little effect on ligand binding.[32]
Rat Mups bind different small chemicals. The most common ligand is 1-Chlorodecane, with 2-methyl-N-phenyl-2-propenamide, hexadecane and 2,6,11-trimethyl decane found to be less prominent.[33] Rat Mups also bind limonene-1,2-epoxide, resulting in a disease of the host's kidney, hyaline-droplet nephropathy, that progresses to cancer. Other species do not develop this disorder because their Mups do not bind that particular chemical.[34] Accordingly, when transgenic mice were engineered to express the rat Mup, their kidneys developed the disease.[35] The Mup found in pigs, named salivary lipocalin (SAL), is expressed in the salivary gland of males where it tightly binds androstenone and androstenol, both pheromones that cause female pigs to assume a mating stance.[1][14]
Isothermal titration calorimetry studies performed with Mups and associated ligands (pyrazines,[36][37] alcohols,[38][39] thiazolines,[40][28] 6-hydroxy-6-methyl-3-heptanone,[41] and N-phenylnapthylamine,[42][43]) revealed an unusual binding phenomena. The active site has been found to be suboptimally hydrated, resulting in ligand binding being driven by enthalpic dispersion forces. This is contrary to most other proteins, which exhibit entropy-driven binding forces from the reorganisation of water molecules. This unusual process has been termed the nonclassical hydrophobic effect.[43]
Pheromones
[edit]
Studies have sought to find the precise function of Mups in pheromone communication. Mup proteins have been shown to promote puberty and accelerate the estrus cycle in female mice, inducing the Vandenbergh and Whitten effects.[38][44] However, in both cases the Mups had to be presented to the female dissolved in male urine, indicating that the protein requires some urinary context to function. In 2007 Mups normally found in male mouse urine were made in transgenic bacteria, and therefore created devoid of the chemicals they normally bind. These Mups were shown to be sufficient to promote aggressive behaviour in males, even in the absence of urine.[19] In addition, Mups made in bacteria were found to activate olfactory sensory neurons in the vomeronasal organ (VNO), a subsystem of the nose known to detect pheromones via specific sensory receptors, of mice and rats.[19][45] Together, this demonstrated that Mup proteins can act as pheromones themselves, independent of their ligands.[46]
Consistent with a role in male-male aggression, adult male mice secrete significantly more Mups into their urine than females, juveniles or castrated male mice. The precise mechanism driving this difference between the sexes is complex, but at least three hormones—testosterone, growth hormone and thyroxine—are known to positively influence the production of Mups in mice.[47] Wild house mouse urine contains variable combinations of four to seven distinct Mup proteins per mouse.[48] Some inbred laboratory mouse strains, such as BALB/c and C57BL/6, also have different proteins expressed in their urine.[20] However, unlike wild mice, different individuals from the same strain express the same protein pattern, an artifact of many generations of inbreeding.[49][50] One unusual Mup is less variable than the others: it is consistently produced by a high proportion of wild male mice and is almost never found in female urine. When this Mup was made in bacteria and used in behavioural testing, it was found to attract female mice. Other Mups were tested but did not have the same attractive qualities, suggesting the male-specific Mup acts as a sex pheromone.[51] Scientists named this Mup darcin (Mup20, Q5FW60) as a humorous reference to Fitzwilliam Darcy, the romantic hero from Pride and Prejudice.[52][53] Taken together, the complex patterns of Mups produced has the potential to provide a range of information about the donor animal, such as gender, fertility, social dominance, age, genetic diversity or kinship.[19][54][55] Wild mice (unlike laboratory mice that are genetically identical and which therefore also have identical patterns of Mups in the urine) have individual patterns of Mup expression in their urine that act as a "barcode" to uniquely identify the owner of a scent mark.[54]
In the house mouse, the major MUP gene cluster provides a highly polymorphic scent signal of genetic identity. Wild mice breeding freely in semi-natural enclosures showed inbreeding avoidance. This avoidance resulted from a strong deficit in successful matings between mice sharing both MUP haplotypes (complete match).[56] In another study, using white-footed mice, it was found that when mice derived from wild populations were inbred, there was reduced survival when such mice were reintroduced into a natural habitat.[57] These findings suggest that inbreeding reduces fitness, and that scent signal recognition has evolved in mice as a means of avoiding inbreeding depression.
Kairomones
[edit]In addition to serving as social cues between members of the same species, Mups can act as kairomones—chemical signals that transmit information between species.[58][59][60] Mice are instinctively afraid of the smell of their natural predators, including cats and rats. This occurs even in laboratory mice that have been isolated from predators for hundreds of generations.[61] When the chemical cues responsible for the fear response were purified from cat saliva and rat urine, two homologous protein signals were identified: Fel d 4 (Felis domesticus allergen 4; Q5VFH6), the product of the cat Mup gene, and Rat n 1 (Rattus norvegicus allergen 1; P02761), the product of the rat Mup13 gene.[59] Mice are fearful of these Mups even when they are made in bacteria, but mutant animals that are unable to detect the Mups showed no fear of rats, demonstrating their importance in initiating fearful behaviour.[58][62] It is not known exactly how Mups from different species initiate disparate behaviours, but mouse Mups and predator Mups have been shown to activate unique patterns of sensory neurons in the nose of recipient mice. This implies the mouse perceives them differently, via distinct neural circuits.[58][59] The pheromone receptors responsible for Mup detection are also unknown, though they are thought be members of the V2R receptor class.[19][59]
Allergens
[edit]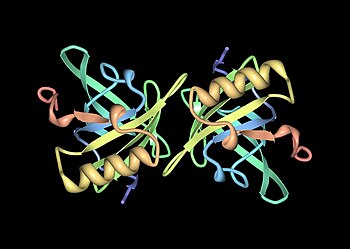
Along with other members of the lipocalin protein family, major urinary proteins can be potent allergens to humans.[64] The reason for this is not known; however, molecular mimicry between Mups and structurally similar human lipocalins has been proposed as a possible explanation.[65] The protein product of the mouse Mup6 and Mup2 genes (previously mistaken as Mup17 due to the similarity among mouse MUPs), known as Mus m 1, Ag1 or MA1,[66] accounts for much of the allergenic properties of mouse urine.[1][67] The protein is extremely stable in the environment; studies have found 95% of inner city homes and 82% of all types of homes in the United States have detectable levels in at least one room.[68][69] Similarly, Rat n 1 is a known human allergen.[64] A US study found its presence in 33% of inner city homes, and 21% of occupants were sensitized to the allergen.[70] Exposure and sensitization to rodent Mup proteins is considered a risk factor for childhood asthma and is a leading cause of laboratory animal allergy (LAA)—an occupational disease of laboratory animal technicians and scientists.[71][72][73][74] One study found that two-thirds of laboratory workers who had developed asthmatic reactions to animals had antibodies to Rat n 1.[75]
Mup genes from other mammals also encode allergenic proteins, for example Fel d 4 is primarily produced in the submandibular salivary gland and is deposited onto dander as the cat grooms itself. A study found that 63% of cat allergic people have antibodies against the protein. Most had higher titres of antibodies against Fel d 4 than against Fel d 1, another prominent cat allergen.[13] Likewise, Equ c 1 (Equus caballus allergen 1; Q95182) is the protein product of a horse Mup gene that is found in the liver, sublingual and submaxillary salivary glands.[1][76] It is responsible for about 80% of the antibody response in patients who are chronically exposed to horse allergens.[76]
Metabolism
[edit]While the detection of Mups excreted by other animals has been well studied, the functional role in the producing animal is less clear. However, in 2009, Mups were shown to be associated with the regulation of energy expenditure in mice. Scientists found that genetically induced obese, diabetic mice produce thirty times less Mup RNA than their lean siblings.[77] When they delivered Mup protein directly into the bloodstream of these mice, they observed an increase in energy expenditure, physical activity and body temperature and a corresponding decrease in glucose intolerance and insulin resistance. They propose that Mups' beneficial effects on energy metabolism occurs by enhancing mitochondrial function in skeletal muscle.[77] Another study found Mups were reduced in diet-induced obese mice. In this case, the presence of Mups in the bloodstream of mice restricted glucose production by directly inhibiting the expression of genes in the liver.[78]
See also
[edit]- Cis-vaccenyl acetate, an insect aggression pheromone
- Major histocompatibility complex, peptides also implicated in individual recognition in mice
- Proteins produced and secreted by the liver
Notes
[edit]- ^ In that year Richard Bright first related kidney disease, later to become known as Bright's disease, with albuminous urine.
References
[edit]- ^ Jump up to: a b c d e f g h i j k l Logan DW, Marton TF, Stowers L (September 2008). "Species specificity in major urinary proteins by parallel evolution". PLOS ONE. 3 (9): e3280. Bibcode:2008PLoSO...3.3280L. doi:10.1371/journal.pone.0003280. PMC 2533699. PMID 18815613.
- ^ Comper WD, Hilliard LM, Nikolic-Paterson DJ, Russo LM (December 2008). "Disease-dependent mechanisms of albuminuria". American Journal of Physiology. Renal Physiology. 295 (6): F1589-600. doi:10.1152/ajprenal.00142.2008. PMID 18579704.
- ^ Lemley KV, Pauling L (1994). "Thomas Addis: 1881–1949". Biographical Memoirs of the National Academy of Sciences. 63: 1–46.
- ^ Addis T (1932). "Proteinuria and cylinduria". Proceedings of the California Academy of Sciences. 2: 38–52.
- ^ Bell ME (September 1933). "Albuminuria in the normal male rat". The Journal of Physiology. 79 (2): 191–3. doi:10.1113/jphysiol.1933.sp003040. PMC 1394952. PMID 16994453.
- ^ Parfentjev IA, Perlzweig WA (1933). "The Composition of the Urine of White Mice". The Journal of Biological Chemistry. 100 (2): 551–55. doi:10.1016/S0021-9258(18)75972-3.
- ^ Jump up to: a b Finlayson JS, Asofsky R, Potter M, Runner CC (August 1965). "Major urinary protein complex of normal mice: origin". Science. 149 (3687): 981–2. Bibcode:1965Sci...149..981F. doi:10.1126/science.149.3687.981. PMID 5827345. S2CID 23007588.
- ^ Jump up to: a b c Roy AK, Neuhaus OW (March 1966). "Identification of rat urinary proteins by zone and immunoelectrophoresis". Proceedings of the Society for Experimental Biology and Medicine. 121 (3): 894–9. doi:10.3181/00379727-121-30917. PMID 4160706. S2CID 41096617.
- ^ Jump up to: a b Roy AK, Neuhaus OW (September 1966). "Proof of the hepatic synthesis of a sex-dependent protein in the rat". Biochimica et Biophysica Acta (BBA) - General Subjects. 127 (1): 82–7. doi:10.1016/0304-4165(66)90478-8. PMID 4165835.
- ^ Held WA, Gallagher JF (April 1985). "Rat alpha 2u-globulin mRNA expression in the preputial gland". Biochemical Genetics. 23 (3–4): 281–90. doi:10.1007/BF00504325. PMID 2409959. S2CID 25646065.
- ^ Gubits RM, Lynch KR, Kulkarni AB, Dolan KP, Gresik EW, Hollander P, et al. (October 1984). "Differential regulation of alpha 2u globulin gene expression in liver, lachrymal gland, and salivary gland". The Journal of Biological Chemistry. 259 (20): 12803–9. doi:10.1016/S0021-9258(18)90817-3. PMID 6208189.
- ^ Shahan K, Denaro M, Gilmartin M, Shi Y, Derman E (May 1987). "Expression of six mouse major urinary protein genes in the mammary, parotid, sublingual, submaxillary, and lachrymal glands and in the liver". Molecular and Cellular Biology. 7 (5): 1947–54. doi:10.1128/MCB.7.5.1947. PMC 365300. PMID 3600653.
- ^ Jump up to: a b Smith W, Butler AJ, Hazell LA, Chapman MD, Pomés A, Nickels DG, et al. (November 2004). "Fel d 4, a cat lipocalin allergen". Clinical and Experimental Allergy. 34 (11): 1732–8. doi:10.1111/j.1365-2222.2004.02090.x. PMID 15544598. S2CID 20266013.
- ^ Jump up to: a b Loebel D, Scaloni A, Paolini S, Fini C, Ferrara L, Breer H, et al. (September 2000). "Cloning, post-translational modifications, heterologous expression and ligand-binding of boar salivary lipocalin". The Biochemical Journal. 350 Pt 2 (Pt 2): 369–79. doi:10.1042/0264-6021:3500369. PMC 1221263. PMID 10947950.
- ^ Beynon RJ, Hurst JL (February 2003). "Multiple roles of major urinary proteins in the house mouse, Mus domesticus". Biochemical Society Transactions. 31 (Pt 1): 142–6. doi:10.1042/BST0310142. PMID 12546672.
- ^ Kurtz DT (1981). "Rat alpha 2u globulin is encoded by a multigene family". Journal of Molecular and Applied Genetics. 1 (1): 29–38. PMID 6180115.
- ^ Hastie ND, Held WA, Toole JJ (June 1979). "Multiple genes coding for the androgen-regulated major urinary proteins of the mouse". Cell. 17 (2): 449–57. doi:10.1016/0092-8674(79)90171-5. PMID 88267. S2CID 20636057.
- ^ Bishop JO, Clark AJ, Clissold PM, Hainey S, Francke U (1982). "Two main groups of mouse major urinary protein genes, both largely located on chromosome 4". The EMBO Journal. 1 (5): 615–20. doi:10.1002/j.1460-2075.1982.tb01217.x. PMC 553096. PMID 6329695.
- ^ Jump up to: a b c d e Chamero P, Marton TF, Logan DW, Flanagan K, Cruz JR, Saghatelian A, et al. (December 2007). "Identification of protein pheromones that promote aggressive behaviour". Nature. 450 (7171): 899–902. Bibcode:2007Natur.450..899C. doi:10.1038/nature05997. PMID 18064011. S2CID 4398766.
- ^ Jump up to: a b c d e Mudge JM, Armstrong SD, McLaren K, Beynon RJ, Hurst JL, Nicholson C, et al. (2008). "Dynamic instability of the major urinary protein gene family revealed by genomic and phenotypic comparisons between C57 and 129 strain mice". Genome Biology. 9 (5): R91. doi:10.1186/gb-2008-9-5-r91. PMC 2441477. PMID 18507838.
- ^ Hurst J, Beynon RJ, Roberts SC, Wyatt TD (2007). Urinary Lipocalins in Rodenta:is there a Generic Model?. Chemical Signals in Vertebrates 11. Springer New York. ISBN 978-0-387-73944-1.
- ^ Cavaggioni A, Mucignat-Caretta C (October 2000). "Major urinary proteins, alpha(2U)-globulins and aphrodisin". Biochimica et Biophysica Acta (BBA) - Protein Structure and Molecular Enzymology. 1482 (1–2): 218–28. doi:10.1016/S0167-4838(00)00149-7. PMID 11058763.
- ^ McFadyen DA, Addison W, Locke J (May 1999). "Genomic organization of the rat alpha 2u-globulin gene cluster". Mammalian Genome. 10 (5): 463–70. doi:10.1007/s003359901024. PMID 10337619. S2CID 1121039.
- ^ Böcskei Z, Groom CR, Flower DR, Wright CE, Phillips SE, Cavaggioni A, et al. (November 1992). "Pheromone binding to two rodent urinary proteins revealed by X-ray crystallography". Nature. 360 (6400): 186–8. Bibcode:1992Natur.360..186B. doi:10.1038/360186a0. PMID 1279439. S2CID 4362015.
- ^ Jump up to: a b Flower DR (August 1996). "The lipocalin protein family: structure and function". The Biochemical Journal. 318 ( Pt 1) (1): 1–14. doi:10.1042/bj3180001. PMC 1217580. PMID 8761444.
- ^ Ganfornina MD, Gutiérrez G, Bastiani M, Sánchez D (January 2000). "A phylogenetic analysis of the lipocalin protein family". Molecular Biology and Evolution. 17 (1): 114–26. doi:10.1093/oxfordjournals.molbev.a026224. PMID 10666711.
- ^ Jump up to: a b Halpern M, Martínez-Marcos A (June 2003). "Structure and function of the vomeronasal system: an update" (PDF). Progress in Neurobiology. 70 (3): 245–318. doi:10.1016/S0301-0082(03)00103-5. PMID 12951145. S2CID 31122845. Archived from the original (PDF) on 2017-11-07.
- ^ Jump up to: a b Timm DE, Baker LJ, Mueller H, Zidek L, Novotny MV (May 2001). "Structural basis of pheromone binding to mouse major urinary protein (MUP-I)". Protein Science. 10 (5): 997–1004. doi:10.1110/ps.52201. PMC 2374202. PMID 11316880.
- ^ Armstrong SD, Robertson DH, Cheetham SA, Hurst JL, Beynon RJ (October 2005). "Structural and functional differences in isoforms of mouse major urinary proteins: a male-specific protein that preferentially binds a male pheromone". The Biochemical Journal. 391 (Pt 2): 343–50. doi:10.1042/BJ20050404. PMC 1276933. PMID 15934926.
- ^ Stowers L, Marton TF (June 2005). "What is a pheromone? Mammalian pheromones reconsidered". Neuron. 46 (5): 699–702. doi:10.1016/j.neuron.2005.04.032. PMID 15924856. S2CID 9354126.
- ^ Hurst JL, Robertson DH, Tolladay U, Beynon RJ (May 1998). "Proteins in urine scent marks of male house mice extend the longevity of olfactory signals". Animal Behaviour. 55 (5): 1289–97. doi:10.1006/anbe.1997.0650. PMID 9632512. S2CID 9879771.
- ^ Darwish Marie A, Veggerby C, Robertson DH, Gaskell SJ, Hubbard SJ, Martinsen L, et al. (February 2001). "Effect of polymorphisms on ligand binding by mouse major urinary proteins". Protein Science. 10 (2): 411–7. doi:10.1110/ps.31701. PMC 2373947. PMID 11266626.
- ^ Rajkumar R, Ilayaraja R, Mucignat C, Cavaggioni A, Archunan G (August 2009). "Identification of alpha2u-globulin and bound volatiles in the Indian common house rat (Rattus rattus)". Indian Journal of Biochemistry & Biophysics. 46 (4): 319–24. PMID 19788064.
- ^ Lehman-McKeeman LD, Caudill D (February 1992). "Biochemical basis for mouse resistance to hyaline droplet nephropathy: lack of relevance of the alpha 2u-globulin protein superfamily in this male rat-specific syndrome". Toxicology and Applied Pharmacology. 112 (2): 214–21. doi:10.1016/0041-008X(92)90190-4. PMID 1371614.
- ^ Lehman-McKeeman LD, Caudill D (November 1994). "d-Limonene induced hyaline droplet nephropathy in alpha 2u-globulin transgenic mice". Fundamental and Applied Toxicology. 23 (4): 562–8. doi:10.1006/faat.1994.1141. PMID 7532604.
- ^ Bingham RJ, Findlay JB, Hsieh SY, Kalverda AP, Kjellberg A, Perazzolo C, et al. (February 2004). "Thermodynamics of binding of 2-methoxy-3-isopropylpyrazine and 2-methoxy-3-isobutylpyrazine to the major urinary protein". Journal of the American Chemical Society. 126 (6): 1675–81. doi:10.1021/ja038461i. PMID 14871097.
- ^ Barratt E, Bingham RJ, Warner DJ, Laughton CA, Phillips SE, Homans SW (August 2005). "Van der Waals interactions dominate ligand-protein association in a protein binding site occluded from solvent water". Journal of the American Chemical Society. 127 (33): 11827–34. doi:10.1021/ja0527525. PMID 16104761.
- ^ Jump up to: a b Mucignat-Caretta C, Caretta A, Cavaggioni A (July 1995). "Acceleration of puberty onset in female mice by male urinary proteins". The Journal of Physiology. 486 ( Pt 2) (Pt 2): 517–22. doi:10.1113/jphysiol.1995.sp020830. PMC 1156539. PMID 7473215.
- ^ Malham R, Johnstone S, Bingham RJ, Barratt E, Phillips SE, Laughton CA, et al. (December 2005). "Strong solute-solute dispersive interactions in a protein-ligand complex". Journal of the American Chemical Society. 127 (48): 17061–7. doi:10.1021/ja055454g. PMID 16316253.
- ^ Sharrow SD, Novotny MV, Stone MJ (May 2003). "Thermodynamic analysis of binding between mouse major urinary protein-I and the pheromone 2-sec-butyl-4,5-dihydrothiazole". Biochemistry. 42 (20): 6302–9. doi:10.1021/bi026423q. PMID 12755635.
- ^ Sharrow SD, Edmonds KA, Goodman MA, Novotny MV, Stone MJ (January 2005). "Thermodynamic consequences of disrupting a water-mediated hydrogen bond network in a protein:pheromone complex". Protein Science. 14 (1): 249–56. doi:10.1110/ps.04912605. PMC 2253314. PMID 15608125.
- ^ Pertinhez TA, Ferrari E, Casali E, Patel JA, Spisni A, Smith LJ (December 2009). "The binding cavity of mouse major urinary protein is optimised for a variety of ligand binding modes". Biochemical and Biophysical Research Communications. 390 (4): 1266–71. doi:10.1016/j.bbrc.2009.10.133. PMID 19878650.
- ^ Jump up to: a b Homans SW (July 2007). "Water, water everywhere--except where it matters?". Drug Discovery Today. 12 (13–14): 534–9. doi:10.1016/j.drudis.2007.05.004. PMID 17631247.
- ^ Marchlewska-koj A, Caretta A, Mucignat-Caretta C, Olejniczak, P (2000). "Stimulation of estrus in female mice by male urinary proteins". Journal of Chemical Ecology. 26 (10): 2355–65. doi:10.1023/A:1005578911652. S2CID 9181177.
- ^ Krieger J, Schmitt A, Löbel D, Gudermann T, Schultz G, Breer H, et al. (February 1999). "Selective activation of G protein subtypes in the vomeronasal organ upon stimulation with urine-derived compounds". The Journal of Biological Chemistry. 274 (8): 4655–62. doi:10.1074/jbc.274.8.4655. PMID 9988702.
- ^ "Aggression protein found in mice". BBC News. 5 December 2007. Retrieved 26 September 2009.
- ^ Knopf JL, Gallagher JF, Held WA (December 1983). "Differential, multihormonal regulation of the mouse major urinary protein gene family in the liver". Molecular and Cellular Biology. 3 (12): 2232–40. doi:10.1128/MCB.3.12.2232. PMC 370094. PMID 6656765.
- ^ Робертсон Д.Х., Херст Дж.Л., Болгар М.С., Гаскелл С.Дж., Бейнон Р.Дж. (1997). «Молекулярная гетерогенность белков мочи в популяциях мыши диких домов». Быстрая связь в масс -спектрометрии . 11 (7): 786–90. Bibcode : 1997rcms ... 11..786r . doi : 10.1002/(SICI) 1097-0231 (19970422) 11: 7 <786 :: AID-RCM876> 3.0.CO; 2-8 . PMID 9161047 .
- ^ Робертсон Д.Х., Кокс К.А., Гаскелл С.Дж., Эвершед Р.П., Бейнон Р.Дж. (май 1996 г.). «Молекулярная гетерогенность в основных белках мочи домашних мышей Mus Musculus» . Биохимический журнал . 316 (Pt 1) (Pt 1): 265–72. doi : 10.1042/bj3160265 . PMC 1217333 . PMID 8645216 .
- ^ Cheetham SA, Smith AL, Armstrong SD, Beynon RJ, Hurst JL (февраль 2009 г.). «Ограниченные различия в основных мочевых белках лабораторных мышей». Физиология и поведение . 96 (2): 253–61. doi : 10.1016/j.physbeh.2008.10.005 . PMID 18973768 . S2CID 20637696 .
- ^ Бреннан Па (июнь 2010 г.). «О запахе сексуального влечения» . BMC Biology . 8 (1): 71. doi : 10.1186/1741-7007-8-71 . PMC 2880966 . PMID 20504292 .
- ^ Робертс С.А., Симпсон Д.М., Армстронг С.Д., Дэвидсон А.Дж., Робертсон Д.Х., Маклин Л. и др. (Июнь 2010 г.). «Дарцин: мужской феромон, который стимулирует женскую память и сексуальное влечение к запаху отдельного мужчины» . BMC Biology . 8 (1): 75. doi : 10.1186/1741-7007-8-75 . PMC 2890510 . PMID 20525243 .
- ^ Московиц С (3 июня 2010 г.). «Биологи узнают, почему мыши идут гага для мочи» . Foxnews.com . Fox News Network . Получено 9 июня 2010 года .
- ^ Jump up to: а беременный Hurst JL, Payne CE, Nevison CM, Marie AD, Humphries RE, Robertson DH, et al. (Декабрь 2001 г.). «Индивидуальное распознавание у мышей, опосредованное основными белками мочи». Природа . 414 (6864): 631–4. Bibcode : 2001natur.414..631H . doi : 10.1038/414631a . PMID 11740558 . S2CID 464644 .
- ^ Том MD, Стокли П., Жюри Ф., Оллиер В.Е., Бейнон Р.Дж., Херст Дж.Л. (апрель 2008 г.). «Прямая оценка генетической гетерозиготности через аромат у мыши» . Текущая биология . 18 (8): 619–23. Bibcode : 2008cbio ... 18..619t . doi : 10.1016/j.cub.2008.03.056 . PMID 18424142 . S2CID 268741 .
- ^ Шерборн А.Л., Том М.Д., Патерсон С., Жюри Ф., Оллиер В.Е., Стокли П. и др. (Декабрь 2007 г.). «Генетическая основа уклонения инбридинга у домашних мышей» . Текущая биология . 17 (23): 2061–6. Bibcode : 2007cbio ... 17.2061s . doi : 10.1016/j.cub.2007.10.041 . PMC 2148465 . PMID 17997307 .
- ^ Jiménez JA, Hughes KA, Alaks G, Graham L, Lacy RC (октябрь 1994 г.). «Экспериментальное исследование депрессии инбридинга в естественной среде обитания». Наука . 266 (5183): 271–3. Bibcode : 1994sci ... 266..271j . doi : 10.1126/science.7939661 . PMID 7939661 .
- ^ Jump up to: а беременный в Papes F, Logan DW, Stowers L (май 2010 г.). «Воомероназальный орган опосредует межвидовое оборонительное поведение посредством обнаружения гомологов белковых феромонов» . Клетка . 141 (4): 692–703. doi : 10.1016/j.cell.2010.03.037 . PMC 2873972 . PMID 20478258 .
- ^ Jump up to: а беременный в дюймовый Родригес I (май 2010 г.). «Химический маппетер» . Клетка . 141 (4): 568–70. doi : 10.1016/j.cell.2010.04.032 . PMID 20478249 . S2CID 13992615 .
- ^ «Почему мыши боятся запаха кошек» . BBC News . 17 мая 2010 г. Получено 18 мая 2010 года .
- ^ Эренберг R (5 июня 2010 г.). «Бой или беги, это в мопе» . Science News . Архивировано с оригинала 12 октября 2012 года . Получено 10 июня 2010 года .
- ^ Bhanoo S (17 мая 2010 г.). «Когда мышь пахнет крысой» . New York Times .
- ^ Lascombe MB, Gregoire C, Poncet P, Tavares GA, Rosinski-Chupin I, Rabillon J, et al. (Июль 2000 г.). «Кристаллическая структура аллергена qu c 1. Димерный липокалин с ограниченными IgE-реактивными эпитопами» . Журнал биологической химии . 275 (28): 21572–7. doi : 10.1074/jbc.m002854200 . PMID 10787420 .
- ^ Jump up to: а беременный Локки Р., Ледфорд Д.К. (2008). «Аллергены млекопитающих». Аллергены и аллерген иммунотерапия . Том 21 клинической аллергии и иммунологии. Informa Health Care. С. 201–218. ISBN 978-1-4200-6197-0 .
- ^ Virtanen T, Zeiler T, Mäntyjärvi R (декабрь 1999 г.). «Важными аллергенами животных являются липокалиновые белки: почему они аллергенные?». Международный архив аллергии и иммунологии . 120 (4): 247–58. doi : 10.1159/000024277 . PMID 10640908 . S2CID 1171463 .
- ^ "Mus M 1 детали аллергена" . www.allergen.org .
- ^ Lorusso Jr, Moffat S, Ohman JL (ноябрь 1986). «Иммунологические и биохимические свойства основного аллергена мочи мыши (Mus M 1)». Журнал аллергии и клинической иммунологии . 78 (5 пт 1): 928–37. doi : 10.1016/0091-6749 (86) 90242-3 . PMID 3097107 .
- ^ Cohn RD, Arbes SJ, Yin M, Jaramillo R, Zeldin DC (июнь 2004 г.). «Национальная распространенность и риск воздействия аллергена мыши в американских домохозяйствах» . Журнал аллергии и клинической иммунологии . 113 (6): 1167–71. doi : 10.1016/j.jaci.2003.12.592 . PMID 15208600 .
- ^ Phipatanakul W, Eggleston PA, Wright EC, Wood RA (декабрь 2000 г.). «Аллерген мыши. I. Распространенность аллергена мыши в городских домах. Национальное кооперативное исследование астмы в городе». Журнал аллергии и клинической иммунологии . 106 (6): 1070–4. doi : 10.1067/mai.2000.110796 . PMID 11112888 .
- ^ Перри Т., Мацуи Е., Мерриман Б., Дуонг Т, Эгглстон П (август 2003 г.). «Распространенность аллергена крыс в домах внутренних города и его связь с сенсибилизацией и заболеваемостью астмы». Журнал аллергии и клинической иммунологии . 112 (2): 346–52. doi : 10.1067/mai.2003.1640 . PMID 12897741 . S2CID 25216587 .
- ^ Wood RA (2001). «Лабораторные аллергены животных» . Ilar Journal . 42 (1): 12–6. doi : 10.1093/ilar.42.1.12 . PMID 11123185 .
- ^ Gaffin JM, Phipatanakul W (апрель 2009 г.). «Роль внутренних аллергенов в развитии астмы» . Современное мнение об аллергии и клинической иммунологии . 9 (2): 128–35. doi : 10.1097/aci.0b013e32832678b0 . PMC 2674017 . PMID 19326507 .
- ^ Pongracic JA, Visnes CM, Gruchalla RS, Evans R, Mitchell HE (июль 2008 г.). «Влияние аллергена мыши и экологического вмешательства грызунов на астму у детей в городке». Анналы аллергии, астмы и иммунологии . 101 (1): 35–41. doi : 10.1016/S1081-1206 (10) 60832-0 . PMID 18681082 .
- ^ Гордон С., Предос Р. (сентябрь 2003 г.). «Профилактика аллергии на лабораторные животные» . Профессиональная медицина . 53 (6): 371–7. doi : 10.1093/occmed/kqg117 . PMID 14514903 .
- ^ Платтс-Миллс Т.А., Лонгботтом Дж., Эдвардс Дж., Кокрофт А., Уилкинс С. (март 1987 г.). «Профессиональная астма и ринит, связанные с лабораторными крысами: сывороточные IgG и IgE -антитела к аллергену мочи крысы». Журнал аллергии и клинической иммунологии . 79 (3): 505–15. doi : 10.1016/0091-6749 (87) 90369-1 . PMID 3819230 .
- ^ Jump up to: а беременный Грегоар С., Розински-Чупин I, Рабиллон Дж., Альзари П.М., Дэвид Б., Дандеу Дж.П. (декабрь 1996 г.). «Клонирование и секвенирование кДНК показывают, что основной аллерген лошадей Equ C1 является гликопротеином суперсемейства липокалина» . Журнал биологической химии . 271 (51): 32951–9. doi : 10.1074/jbc.271.51.32951 . PMID 8955138 .
- ^ Jump up to: а беременный Hui X, Zhu W, Wang Y, Lam KS, Zhang J, Wu D, et al. (Май 2009 г.). «Основной белок-1 мочи увеличивает расходы энергии и повышает непереносимость глюкозы за счет усиления митохондриальной функции в скелетных мышцах мышей с диабетом» . Журнал биологической химии . 284 (21): 14050–7. doi : 10.1074/jbc.m109.001107 . PMC 2682853 . PMID 19336396 .
- ^ Zhou Y, Jiang L, Rui L (апрель 2009 г.). «Идентификация MUP1 в качестве регулятора для метаболизма глюкозы и липидов у мышей» . Журнал биологической химии . 284 (17): 11152–9. doi : 10.1074/jbc.m900754200 . PMC 2670120 . PMID 19258313 .
Внешние ссылки
[ редактировать ]- Аромат грызуна , почему файлы - наука, стоящая за новостями
- Сигналы страха от хищников на YouTube , видео, описывающее исследование, которое определило, что Mups были кайромонами