Аспарагин -синтетаза
| аспарагин -синтетаза | |||
|---|---|---|---|
 | |||
| Идентификаторы | |||
| Символ | Ростка | ||
| Альт. символы | 11as, рост | ||
| Ген NCBI | 440 | ||
| HGNC | 753 | ||
| Омим | 108370 | ||
| Refseq | NM_001673 | ||
| Uniprot | P08243 | ||
| Другие данные | |||
| EC number | 6.3.5.4 | ||
| Locus | Chr. 7 q21-q31 | ||
| |||
Аспарагиновая синтетаза (или аспартат-аммония лигаза ) представляет собой в основном цитоплазматический фермент, который генерирует аспарагин из аспартата . [ 1 ] Эта реакция амидирования аналогична той, которая способствует глютаминовой синтетазе . Фермент вездесущ в своем распределении в органах млекопитающих, но базальная экспрессия относительно низкая в тканях, кроме экзокринной поджелудочной железы. [ 2 ]
Присутствие аспарагиновой синтетазы в определенных штаммах лейкоза было связано, чтобы быть значительным фактором устойчивости к химиотерапии, особенно с химиотерапевтическим препаратом L-аспарагиназы . [ 3 ]
Структура
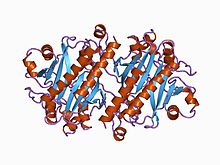
[ редактировать ]Escherichia coli, полученная для аспарагиновой синтетазы, представляет собой димерный белок с каждой субъединицей, складывающейся в два различных домена. [ 4 ] N-концевая область состоит из двух слоев шестицепочечных антипараллельных β -листов, между которыми является активным сайтом, ответственным за гидролиз глутамина . [4] The C-terminal domain consists of a five-stranded parallel β-sheet flanked on either side by α-helices. This domain is responsible for the binding of both Mg2+ATP and aspartate.[4] These two active sites are connected by a tunnel lined primarily with backbone atoms and hydrophobic, nonpolar amino acid residues.[4]
Structural characterization of asparagine synthetase from mammalian sources have been difficult due to the low abundance and instability of the enzyme during purification procedures.[5]
Mechanism
[edit]Using information from Escherichia coli derived asparagine synthetase, some basic mechanisms of the enzyme have been understood.[5] The N-terminal active site catalyzes glutamine hydrolysis to yield glutamate and ammonia.[5] The C-terminal active site catalyzes activation of the side-chain carboxylate of aspartate to form an electrophilic intermediate, β-aspartyl-AMP (βAspAMP) 1, and inorganic pyrophosphate (PPi).[5] The tunnel that links the two active sites allows for the passage of an ammonia molecule to act as a common intermediate to couple the two half-reactions carried out in the independent active sites of the enzyme.[5] Thus, after being released in, and channeled from, the glutaminase site, the ammonia molecule attacks the bound βAspAMP 1 to give asparagine and AMP via a tetrahedral intermediate.[5]
Function
[edit]In plants, inorganic nitrogen is taken up from the environment in forms of nitrate or ammonium.[6] Assimilation of this nitrogen into asparagine for use in nitrogen recycling, transport, and storage is an essential process for plant development, making asparagine synthetase vital to maintaining these asparagine reserves.[6] Specific events in development which depend on asparagine synthetase are nitrogen mobilization in germinating seeds, nitrogen recycling and flow in vegetative cells in response to biotic and abiotic stresses, and nitrogen remobilization from source to sink organs.[6]
In mammals, asparagine synthetase expression has been found to be linked to cell growth, and its mRNA content is linked to changes in the cell cycle.[5] Hamster BHK ts11 cells produce an inactive asparagine synthetase enzyme, and this loss of asparagine synthetase activity directly led to cell cycle arrest in the cells as a consequence of a depletion of cellular asparagine.[5] Upregulation of asparagine synthetase mRNA was observed as well in these hamster cells.[5] Other experiments demonstrated that quiescent rat thyroid cells entering S phase as a result of thyroid-stimulating hormone treatment was matched with a concurrent increase in asparagine synthetase mRNA content.[5]
Classes
[edit]There seem to be two major groups of asparagine synthetase:[7][6]
- Majority of prokaryotic isolated enzymes (asnA) utilize ammonia as the sole nitrogen source.[7][6]
- Eukaryotic isolated and some prokaryotic isolated enzymes (asnB) utilize glutamine as the preferred nitrogen source, although these enzymes can also employ ammonia as an alternate substrate.[7][6] The human glutamine-dependent AS is encoded by a single gene located in region q21.3 on chromosome 7.[8] The lack of ammonia-dependent asparagine synthetase in eukaryotes is presumably because of the need to maintain cellular concentrations of ammonia at very low levels.[7]
Clinical significance
[edit]Cancer
[edit]Leukemia
[edit]Cancerous cells exhibit rapid growth and cell division and subsequently have an increased nutritional need.[5] The particularly low-level expression of asparagine synthetase in primary acute lymphoblastic leukemia (ALL) and numerous ALL cell lines, as compared to that of normal cells, makes asparagine depletion an effective method of treatment due to the cells' unusual dependency on circulating serum asparagine as a necessary nutrition for growth.[2][5] As a result, L-asparaginase is a common chemotherapy drug utilized in the treatment of ALL and may have applications in other asparagine synthetase negative cancers, such as lymphomas, due to its aspariginase activity to deplete serum asparagine.[9] This depletion of serum asparagine leads to a subsequent rapid efflux of cellular asparagine, which is immediately acted upon and destroyed by the L-asparaginase as well.[5] Due to the transient response from these susceptible cancers in reaction to the asparagine depletion, tumor growth is significantly inhibited due to nutritional deficiency.[5][3]
Most somatic cells express sufficient basal amounts of asparagine synthetase to counteract this asparagine starvation and survive the effects of L-asparaginase.[2][3][5] In addition, these normal cells are able to upregulate their expression of asparagine synthetase in response to the asparagine depletion, further countering some of the toxic effects of the medication on normal cell activity, a desirable trait for chemotherapy drugs.[2][3][5]
However, the opposite effect is visible in cases of asparaginase resistant cancers.[3] In these resistant cancers, the effect of blood asparagine depletion through L-asparaginase instead leads to significant asparagine synthetase overexpression to compensate, effectively nullifying the effect of the chemotherapy drug.[3] For example, in mouse models, 24 hours after exposure to L-asparaginase, tumors resistant to the depletion responded with 5- to 19-fold increases in asparagine synthetase expression.[10] These resistant tumors also inherently express higher levels of asparagine synthetase activity, even without the application of L-asparaginase to drive further expression.[11] Similar trends are often seen in human studies as well, with high levels of asparagine synthetase activity being detected in asparaginase-resistant cases of treatment as compared with the negligible activity of susceptible cases.[12] As seen in in vitro studies of resistant human leukemia cell lines, even six weeks after the removal of asparagine depleting factors, the increased level of expression of asparagine synthetase failed to return to a basal state, instead remaining elevated and retaining continued drug resistance.[13]
While the mechanisms underlying the sustained over-expression of ASNS have not been reported in these studies, transcriptome profiling of two T-ALL patients that have relapsed after L-asparaginase treatment revealed a recurrent promoter swap with KMT2E leading to ASNS over-expression and L-asparaginase resistance.[14] It has been further demonstrated in mouse model systems that repeated subculturing of L-asparaginase sensitive tumor cells in sublethal concentrations of L-asparaginase could eventually make them resistant, a potential worry of lower dose chemotherapy treatments effectively encouraging resistant cell development.[15]
Potential biomarker for ovarian cancer
[edit]A correlation between L-asparaginase efficacy and asparagine synthetase protein levels in a number of human ovarian cell lines has been observed.[16] As mentioned above, this result confirmed similar observations in human leukemia cell lines.[16] Hence asparagine synthetase might be used as a biomarker in ovarian cancer screening and potential treatment.[16]
Potential role in solid tumor metastasis
[edit]An epithelial to mesenchymal transition was mimicked in metastatic cells by adapting PC-3 prostate cancer cells from adherent to suspension culture and then examined to investigate changes in gene expression concurrent with this adaption to suspension.[17] It was found that the asparagine synthetase expression was sixfold greater in the suspension cells than in the adherent cells.[17] In xenografts from a human breast cancer cell line in an established metastatic mouse model,[2][18] asparagine synthetase was elevated in circulating tumor cells isolated from the mouse blood compared with the parental cell line.[2][18] When these circulating tumor cells were returned to an in vitro culture and exposed to hypoxia, they showed higher basal expression and greater induction of asparagine synthetase than their parental cell line.[2][18] These circulating tumor cells were also found to have an increased capacity for colony formation in soft agar assays under hypoxic conditions and grew faster when reimplanted as xenografts.[2][18] The increased prevalence of asparaginase synthetase in the metastatic cells suggests that its activity may be beneficial for circulating tumor cell survival.[2][18]
Trivia
[edit]Guinea pigs have some of the highest levels of naturally expressing asparagine synthetase due to the fact that their serum inherently containing detectable levels of L-asparaginase.[10]
References
[edit]- ^ Hutson RG, Kitoh T, Moraga Amador DA, Cosic S, Schuster SM, Kilberg MS (May 1997). "Amino acid control of asparagine synthetase: relation to asparaginase resistance in human leukemia cells". The American Journal of Physiology. 272 (5 Pt 1): C1691-9. doi:10.1152/ajpcell.1997.272.5.C1691. PMID 9176161.
- ^ Jump up to: a b c d e f g h i Balasubramanian MN, Butterworth EA, Kilberg MS (April 2013). "Asparagine synthetase: regulation by cell stress and involvement in tumor biology". American Journal of Physiology. Endocrinology and Metabolism. 304 (8): E789-99. doi:10.1152/ajpendo.00015.2013. PMC 3625782. PMID 23403946.
- ^ Jump up to: a b c d e f Prager MD, Bachynsky N (April 1968). "Asparagine synthetase in asparaginase resistant and susceptible mouse lymphomas". Biochemical and Biophysical Research Communications. 31 (1): 43–7. doi:10.1016/0006-291x(68)90028-4. PMID 4869945.
- ^ Jump up to: a b c d Larsen TM, Boehlein SK, Schuster SM, Richards NG, Thoden JB, Holden HM, Rayment I (December 1999). "Three-dimensional structure of Escherichia coli asparagine synthetase B: a short journey from substrate to product". Biochemistry. 38 (49): 16146–57. doi:10.1021/bi9915768. PMID 10587437.
- ^ Jump up to: a b c d e f g h i j k l m n o p Richards NG, Kilberg MS (July 2006). "Asparagine synthetase chemotherapy". Annual Review of Biochemistry. 75: 629–54. doi:10.1146/annurev.biochem.75.103004.142520. PMC 3587692. PMID 16756505.
- ^ Jump up to: a b c d e f Gaufichon L, Reisdorf-Cren M, Rothstein SJ, Chardon F, Suzuki A (September 2010). "Biological functions of asparagine synthetase in plants". Plant Science. 179 (3): 141–153. doi:10.1016/j.plantsci.2010.04.010.
- ^ Jump up to: a b c d Richards NG, Schuster SM (November 1998). "Mechanistic issues in asparagine synthetase catalysis". Advances in Enzymology and Related Areas of Molecular Biology. Vol. 72. pp. 145–98. doi:10.1002/9780470123188.ch5. ISBN 9780470123188. PMID 9559053.
- ^ Heng HH, Shi XM, Scherer SW, Andrulis IL, Tsui LC (1994). "Refined localization of the asparagine synthetase gene (ASNS) to chromosome 7, region q21.3, and characterization of the somatic cell hybrid line 4AF/106/KO15". Cytogenetics and Cell Genetics. 66 (2): 135–8. doi:10.1159/000133685. hdl:10722/42532. PMID 7904551.
- ^ Chan WK, Lorenzi PL, Anishkin A, Purwaha P, Rogers DM, Sukharev S, Rempe SB, Weinstein JN (June 2014). "The glutaminase activity of L-asparaginase is not required for anticancer activity against ASNS-negative cells". Blood. 123 (23): 3596–606. doi:10.1182/blood-2013-10-535112. PMC 4047499. PMID 24659632.
- ^ Jump up to: a b Prager MD, Bachynsky N (September 1968). "Asparagine synthetase in normal and malignant tissues: correlation with tumor sensitivity to asparaginase". Archives of Biochemistry and Biophysics. 127 (1): 645–54. doi:10.1016/0003-9861(68)90273-7. PMID 4880551.
- ^ Horowitz B, Madras BK, Meister A, Old LJ, Boyes EA, Stockert E (May 1968). "Asparagine synthetase activity of mouse leukemias". Science. 160 (3827): 533–5. Bibcode:1968Sci...160..533H. doi:10.1126/science.160.3827.533. PMID 5689413. S2CID 39734239.
- ^ Haskell CM, Canellos GP (October 1969). "l-asparaginase resistance in human leukemia--asparagine synthetase". Biochemical Pharmacology. 18 (10): 2578–80. doi:10.1016/0006-2952(69)90375-x. PMID 4935103.
- ^ Aslanian AM, Fletcher BS, Kilberg MS (July 2001). "Asparagine synthetase expression alone is sufficient to induce l-asparaginase resistance in MOLT-4 human leukaemia cells". The Biochemical Journal. 357 (Pt 1): 321–8. doi:10.1042/bj3570321. PMC 1221958. PMID 11415466.
- ^ Khater F, Lajoie M, Langlois S, Healy J, Cellot S, Richer C, Beaulieu P, St-Onge P, Sailloir V, Minden M, Marzouki M, Krajinovic M, Bittencourt H, Sinnett D (2017). "KMT2E-ASNS: a novel relapse-specific fusion gene in early T-cell precursor acute lymphoblastic leukemia". Blood. 129 (12): 1729–1732. doi:10.1182/blood-2016-10-744219. PMC 5374844. PMID 28069604.
- ^ Андрулис Ил, Чен Дж., Рэй П.Н. (июль 1987 г.). «Выделение кДНК человека для аспарагиновой синтетазы и экспрессии в клетках саркомы Дженсена крысы» . Молекулярная и клеточная биология . 7 (7): 2435–43. doi : 10.1128/mcb.7.7.2435 . PMC 365375 . PMID 2886907 .
- ^ Jump up to: а беременный в Lorenzi PL, Weinstein JN (январь 2009 г.). «Аспарагин -синтетаза: новый потенциальный биомаркер при раке яичников» . News News & Perspectives . 22 (1): 61–4. doi : 10.1358/dnp.2009.22.1.1303820 . PMC 4096155 . PMID 19209300 .
- ^ Jump up to: а беременный Patrikainen L, Porvari K, Kurkela R, Hirvikoski P, Soini Y, Vihko P (февраль 2007 г.). «Профилирование экспрессии вариантов клеточной линии PC-3 и сравнение уровней транскрипта MIC-1 в доброкачественной и злокачественной простате». Европейский журнал клинических исследований . 37 (2): 126–33. doi : 10.1111/j.1365-2362.2007.01763.x . PMID 17217378 . S2CID 29946047 .
- ^ Jump up to: а беременный в дюймовый и Ameri K, Luong R, Zhang H, Powell AA, Montgomery KD, Espinosa I, Bouley DM, Harris AL, Джеффри С.С. (февраль 2010 г.). «Циркулирующие опухолевые клетки демонстрируют измененный ответ на гипоксию и агрессивный фенотип» . Британский журнал рака . 102 (3): 561–9. doi : 10.1038/sj.bjc.6605491 . PMC 2805847 . PMID 20051957 .
Внешние ссылки
[ редактировать ]- Аспарагин+синтетаза в Национальной медицинской библиотеке Медицинской библиотеки США (Mesh)