Кофейная кислота

| |

| |

| |
| Names | |
|---|---|
| IUPAC names
3-(3,4-Dihydroxyphenyl)-2-propenoic acid
3,4-Dihydroxycinnamic acid trans-Caffeate 3,4-Dihydroxy-trans-cinnamate (E)-3-(3,4-dihydroxyphenyl)-2-propenoic acid 3,4-Dihydroxybenzeneacrylicacid 3-(3,4-Dihydroxyphenyl)-2-propenoic acid | |
| Preferred IUPAC name
(2E)-3-(3,4-Dihydroxyphenyl)prop-2-enoic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| 1954563 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.005.784 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| Properties | |
| C9H8O4 | |
| Molar mass | 180.16 g/mol |
| Density | 1.478 g/cm3 |
| Melting point | 223 to 225 °C (433 to 437 °F; 496 to 498 K) |
| UV-vis (λmax) | 327 nm and a shoulder at c. 295 nm in acidified methanol[1] |
| Hazards | |
| GHS labelling: | |
 
| |
| Warning | |
| H315, H319, H335, H351, H361 | |
| P201, P202, P261, P264, P271, P280, P281, P302+P352, P304+P340, P305+P351+P338, P308+P313, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Related compounds
|
Chlorogenic acid Cichoric acid Coumaric acid Quinic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Кофеиновая кислота является органическим соединением с формулой HO) 2 C 6 H 3 CH = CHCO 2 H. ( Это полифенол. Это желтое твердое вещество. Структурно он классифицируется как гидроксициннамическая кислота . Молекула состоит из фенольных и акриловых функциональных групп. Он обнаруживается во всех растениях как промежуточное соединение в биосинтезе лигнина и его , одного из основных компонентов биомассы остатков. [ 2 ] Это не связано с кофеином .
Природные события
[ редактировать ]Кофеиновая кислота можно найти в коре эвкалипта Globulus [ 3 ] Зерновый оркес [ 4 ] Его также можно найти в пресной папоротнике Salvinia solesta [ 5 ] и в грибах Phellinus linteus . [ 6 ]
Во -первых, в еде
[ редактировать ]Free caffeic acid can be found in a variety of beverages, including brewed coffee at 63.1-96.0 mg per 100 ml[7] and red wine at 2 mg per 100 ml.[8] It is found at relatively high levels in herbs of the mint family, especially thyme, sage and spearmint (at about 20 mg per 100 g), and in spices, such as Ceylon cinnamon and star anise (at about 22 mg per 100 g). Caffeic acid occurs at moderate levels in sunflower seeds (8 mg per 100 g), apple sauce, apricots and prunes (at about 1 mg per 100 g).[9] It occurs at remarkably high levels in black chokeberry (141 mg per 100 g).[10] It is also quite high in the South American herb yerba mate (150 mg per 100 g based on thin-layer chromatography densitometry[11] and HPLC [12]). It is also found at lower levels in barley and rye.[13]
Biosynthesis
[edit]Caffeic acid is biosynthesized by hydroxylation of coumaroyl ester of quinic acid (esterified through a side chain alcohol). This hydroxylation produces the caffeic acid ester of shikimic acid, which converts to chlorogenic acid. It is the precursor to ferulic acid, coniferyl alcohol, and sinapyl alcohol, all of which are significant building blocks in lignin.[2] The transformation to ferulic acid is catalyzed by the enzyme caffeate O-methyltransferase.
Caffeic acid and its derivative caffeic acid phenethyl ester (CAPE) are produced in many kinds of plants.[14][15][16]
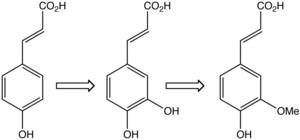
In plants, caffeic acid (middle) is formed from 4-hydroxycinnamic acid (left) and is transformed to ferulic acid.
Dihydroxyphenylalanine ammonia-lyase was presumed to use 3,4-dihydroxy-L-phenylalanine (L-DOPA) to produce trans-caffeate and NH3. However, the EC number for this purported enzyme was deleted in 2007, as no evidence has emerged for its existence.[17]
Biotransformation
[edit]Caffeate O-methyltransferase is an enzyme responsible for the transformation of caffeic acid into ferulic acid.
Caffeic acid and related o-diphenols are rapidly oxidized by o-diphenol oxidases in tissue extracts.[18]
Biodegradation
[edit]Caffeate 3,4-dioxygenase is an enzyme that uses caffeic acid and oxygen to produce 3-(2-carboxyethenyl)-cis,cis-muconate.
Caffeic acid is susceptible to autoxidation. Glutathione and thiol compounds (cysteine, thioglycolic acid or thiocresol) or ascorbic acid have a protective effect on browning and disappearance of caffeic acid.[19] This browning is due to the conversion of o-diphenols into reactive o-quinones. Chemical oxidation of caffeic acid in acidic conditions using sodium periodate leads to the formation of dimers with a furan structure (isomers of 2,5-(3′,4′-dihydroxyphenyl)tetrahydrofuran 3,4-dicarboxylic acid).[20] Caffeic acid can also be polymerized using the horseradish peroxidase/H2O2 oxidizing system.[21]
Glycosides
[edit]3-O-caffeoylshikimic acid (dactylifric acid) and its isomers, are enzymic browning substrates found in dates (Phoenix dactylifera fruits).[22]
Pharmacology
[edit]Caffeic acid has a variety of potential pharmacological effects in in vitro studies and in animal models, and the inhibitory effect of caffeic acid on cancer cell proliferation by an oxidative mechanism in the human HT-1080 fibrosarcoma cell line has recently been established.[23]
Caffeic acid is an antioxidant in vitro and also in vivo.[16] Caffeic acid also shows immunomodulatory and anti-inflammatory activity. Caffeic acid outperformed the other antioxidants, reducing aflatoxin production by more than 95 percent. The studies are the first to show that oxidative stress that would otherwise trigger or enhance Aspergillus flavus aflatoxin production can be stymied by caffeic acid. This opens the door to use as a natural fungicide by supplementing trees with antioxidants.[24]
Studies of the carcinogenicity of caffeic acid have mixed results. Some studies have shown that it inhibits carcinogenesis, and other experiments show carcinogenic effects.[25] Oral administration of high doses of caffeic acid in rats has caused stomach papillomas.[25] In the same study, high doses of combined antioxidants, including caffeic acid, showed a significant decrease in growth of colon tumors in those same rats. No significant effect was noted otherwise. Caffeic acid is listed under some Hazard Data sheets as a potential carcinogen,[26] as has been listed by the International Agency for Research on Cancer as a Group 2B carcinogen ("possibly carcinogenic to humans").[27] More recent data show that bacteria in the rats' guts may alter the formation of metabolites of caffeic acid.[28][29] Other than caffeic acid being a thiamine antagonist (antithiamine factor), there have been no known ill effects of caffeic acid in humans. Also, caffeic acid treatment attenuated lipopolysaccharide (LPS)-induced sickness behaviour in experimental animals by decreasing both peripheral and central cytokine levels along with oxidative stress inflicted by LPS.[30]
Other uses
[edit]Caffeic acid may be the active ingredient in caffenol, a do-it-yourself black-and-white photographic developer made from instant coffee.[31] The developing chemistry is similar to that of catechol or pyrogallol.[32]
It is also used as a matrix in MALDI mass spectrometry analyses.[33]
Isomers
[edit]Isomers with the same molecular formula and in the hydroxycinammic acids family are:
- Umbellic acid (2,4-dihydroxycinnamic acid)
- 2,3-Dihydroxycinnamic acid
- 2,5-Dihydroxycinnamic acid
References
[edit]- ^ Gould, Kevin S.; Markham, Kenneth R.; Smith, Richard H.; Goris, Jessica J. (2000). "Functional role of anthocyanins in the leaves of Quintinia serrata A. Cunn". Journal of Experimental Botany. 51 (347): 1107–1115. doi:10.1093/jexbot/51.347.1107. PMID 10948238.
- ^ Jump up to: a b Boerjan, Wout; Ralph, John; Baucher, Marie (2003). "Lignin biosynthesis". Annual Review of Plant Biology. 54: 519–546. doi:10.1146/annurev.arplant.54.031902.134938. PMID 14503002.
- ^ Santos, Sónia A. O.; Freire, Carmen S. R.; Domingues, M. Rosário M.; Silvestre, Armando J. D.; Pascoal Neto, Carlos (2011). "Characterization of Phenolic Components in Polar Extracts of Eucalyptus globulus Labill. Bark by High-Performance Liquid Chromatography–Mass Spectrometry". Journal of Agricultural and Food Chemistry. 59 (17): 9386–9393. doi:10.1021/jf201801q. PMID 21761864.
- ^ Khoo, Cheang S.; Sullivan, Shaun; Kazzem, Magdy; Lamin, Franklin; Singh, Swastika; Nang, Marnilar; Low, Mitchell; Suresh, Harsha; Lee, Samiuela (2014). "The Liquid Chromatographic Determination of Chlorogenic and Caffeic Acids in Xu Duan (Dipsacus asperoides) Raw Herb". ISRN Analytical Chemistry. 2014: 1–6. doi:10.1155/2014/968314.
- ^ Choudhary, M. Iqbal; Naheed, Nadra; Abbaskhan, Ahmed; Musharraf, Syed Ghulam; Siddiqui, Hina; Atta-Ur-Rahman (2008). "Phenolic and other constituents of fresh water fern Salvinia molesta". Phytochemistry. 69 (4): 1018–1023. Bibcode:2008PChem..69.1018C. doi:10.1016/j.phytochem.2007.10.028. PMID 18177906.
- ^ Lee, Y.-S.; Kang, Y.-H.; Jung, J.-Y.; Lee, Sanghyun; Ohuchi, Kazuo; Shin, Kuk Hyun; Kang, Il-Jun; Park, Jung Han Yoon; Shin, Hyun-Kyung; Soon, Sung (October 2008). "Protein glycation inhibitors from the fruiting body of Phellinus linteus". Biological & Pharmaceutical Bulletin. 31 (10): 1968–1972. doi:10.1248/bpb.31.1968. PMID 18827365.
- ^ Pirjo, Mittila; Kumpulainen, Jorma (19 June 2002). "Determination of free and total phenolic acids in plant-derived foods by HPLC with diode-array detection". J Agric Food Chem. 50 (13): 3660–7. doi:10.1021/jf020028p. PMID 12059140.
- ^ "Showing all foods in which the polyphenol Caffeic acid is found - Phenol-Explorer".
- ^ "Caffeic acid". Iarc Monographs on the Evaluation of Carcinogenic Risks to Humans. 56: 115–134. 1993. PMC 7681336. PMID 8411618.
- ^ Zheng, Wei; Wang, Shiow Y (15 January 2003). "Oxygen radical absorbing capacity of phenolics in blueberries, cranberries, chokeberries, and lingonberries". J Agric Food Chem. 51 (2): 502–9. doi:10.1021/jf020728u. PMID 12517117.
- ^ Bojić, Mirza; Haas, Vicente Simon; Šarić, Darija; Maleš, Željan (4 April 2018). "Determination of Flavonoids, Phenolic Acids, and Xanthines in Mate Tea (Ilex paraguariensis St.-Hil.)". Journal of Analytical Methods in Chemistry. 2013: 658596. doi:10.1155/2013/658596. PMC 3690244. PMID 23841023.
- ^ Berté, Kleber A. S. (2011). "Chemical Composition and Antioxidant Activity of Yerba-Mate (Ilex paraguariensis A. St.-Hil., Aquifoliaceae) Extract as Obtained by Spray Drying". Journal of Agricultural and Food Chemistry. 59 (10): 5523–5527. doi:10.1021/jf2008343. PMID 21510640.
- ^ Quinde-Axtell, Zory; Baik, Byung-Kee (2006). "Phenolic Compounds of Barley Grain and Their Implication in Food Product Discoloration". J. Agric. Food Chem. 54 (26): 9978–9984. doi:10.1021/jf060974w. PMID 17177530.
- ^ "Red Clover Benefits & Information". indigo-herbs.co.uk. Retrieved 4 April 2018.
- ^ "Dr. Duke's Phytochemical and Ethnobotanical Databases". Archived from the original on 2000-12-05.
- ^ Jump up to: a b Olthof, M. R.; Hollman, P. C.; Katan, M. B. (January 2001). "Chlorogenic acid and caffeic acid are absorbed in humans". J. Nutr. 131 (1): 66–71. doi:10.1093/jn/131.1.66. PMID 11208940.
- ^ "EC 4.3.1.11". www.chem.qmul.ac.uk. Archived from the original on 3 March 2016. Retrieved 4 April 2018.
- ^ Pierpoint, WS (1969). « o -quinones образуются в растительных экстрактах. Их реакции с аминокислотами и пептидами» . Биохимия. Дж . 112 (5): 609–616. doi : 10.1042/bj1120609 . PMC 1187763 . PMID 4980678 .
- ^ Cilliers, Johannes JL; Singleton, Vernon L. (1990). «Аутокисление кофеиновой кислоты и эффекты тиолов». J. Agric. Пищевая химия . 38 (9): 1789–1796. doi : 10.1021/jf00099a002 .
- ^ Фулгранд, Элен; Cheminat, Энни; Brouillard, Raymond; Cheynier, Véronique (1994). «Характеристика соединений, полученных химическим окислением кофейной кислоты в кислотных условиях». Фитохимия . 35 (2): 499–505. Bibcode : 1994pchem..35..499f . doi : 10.1016/s0031-9422 (00) 94790-3 .
- ^ Сюй, Пэн; Уяма, Хироши; Уиттен, Джеймс Э.; Кобаяши, Широ; Каплан, Дэвид Л. (2005). «Катализируемая пероксидазой полимеризация кофеиновой кислоты на поверхности». J. Am. Химический Соц 127 (33): 11745–11753. doi : 10.1021/ja051637r . PMID 16104752 .
- ^ Майер, вице -президент; Метцлер, DM; Huber, AF (1964). «3 -o -Caffeoylshikimic кислота (дактилифкистная кислота) и ее изомеры, новый класс субстратов ферментативных подручков». Биохимическая и биофизическая исследовательская коммуникация . 14 (2): 124–128. doi : 10.1016/0006-291x (64) 90241-4 . PMID 5836492 .
- ^ Rajendra Prasad, N.; Картикеян, А.; Karthikeyan, S.; Редди, Б.В. (март 2011 г.). «Ингибирующее влияние кофейной кислоты на пролиферацию раковых клеток путем окислительного механизма в клеточной линии фибросаркомы HT-1080 человека». Мол -клеточная биохимия . 349 (1–2): 11–19. doi : 10.1007/s11010-010-0655-7 . PMID 21116690 . S2CID 28014579 .
- ^ "Новый афлатоксин -боец орехов: кофеиновая кислота?" Полем
- ^ Jump up to: а беременный Хироз, М.; Тайдада, у.; Танака, Х.; Tamano, S.; Като, Т.; Ширай Т. (1998). «Канцерогенность антиоксидантов BHA, кофеиновой кислоты, сезамола, 4-метоксифенола и катехоля в низких дозах, отдельно или в комбинации, а также модуляция их воздействия в среднесрочной модели многоорганного канцерогенеза крысы» . Канцерогенез . 19 (1): 207–212. doi : 10.1093/карцин/19.1.207 . PMID 9472713 .
- ^ «Кофеиновая кислота» . Сводка и оценка IARC . 1993.
- ^ «Агенты, классифицированные по монографиям IARC» (PDF) . iarc.fr. Международное агентство по исследованиям рака . Архивировано из оригинала (PDF) 25 октября 2011 года . Получено 4 апреля 2018 года .
- ^ Peppercorn, MA; Goldman, P. (1972). «Метаболизм кофеиновой кислоты гнотобиотическими крысами и их кишечные бактерии» . Труды Национальной академии наук . 69 (6): 1413–1415. Bibcode : 1972pnas ... 69.1413p . doi : 10.1073/pnas.69.6.1413 . PMC 426714 . PMID 4504351 .
- ^ Gonthier, M.-P.; Верни, М.-А.; Бессон, C.; Rémésy, C.; Скальберт А. (1 июня 2003 г.). «Биододоступность хлорогеновой кислоты в значительной степени зависит от его метаболизма кишечной микрофлорой у крыс» . Журнал питания . 133 (6): 1853–1859. doi : 10.1093/jn/133.6.1853 . PMID 12771329 .
- ^ Басу, Маллик С; и др. (3 сентября 2016 г.). «Кофеиновая кислота ослабляет липополисахарид-индуцированное поведение по болезни и нейровоспаление у мышей». Нейробиологические буквы . 632 : 218–223. doi : 10.1016/j.neulet.2016.08.044 . PMID 27597761 . S2CID 5361129 .
- ^ "Caffenol-CM, рецепт" . Блог кафенола . 2 марта 2010 г.
- ^ Уильямс, Скотт. «Использование для этой последней чашки кофе: развитие фильмов и бумаги» . Техническая фотографическая химия 1995 класс . Департамент визуализации и фотографических технологий, Школа фотографических искусств и наук, Технологический институт Рочестера.
- ^ Бивис, RC; Chait, BT (декабрь 1989). «Производные коричневой кислоты как матрицы для ультрафиолетовой масс -спектрометрии белков». Быстрое общение. Масс -спектр . 3 (12): 432–435. Bibcode : 1989rcms .... 3..432b . doi : 10.1002/rcm.1290031207 . PMID 2520223 .
Внешние ссылки
[ редактировать ]- «Химическая земля» . Кофеиновая кислота как карбоциклическая карбоновая кислота .

