Апоморфин
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Apokyn, Kynmobi |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a604020 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Subcutaneous injection (SQ), sublingual |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 100% following injection |
| Protein binding | ~50% |
| Metabolism | Liver, phase II |
| Onset of action | 10–20 min |
| Elimination half-life | 40 minutes |
| Duration of action | 60–90 min |
| Excretion | Liver |
| Identifiers | |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.327 |
| Chemical and physical data | |
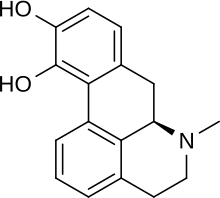
| Formula | C17H17NO2 |
| Molar mass | 267.328 g·mol−1 |
| 3D model (JSmol) | |
| Data page | |
| Apomorphine (data page) | |
| | |
Апоморфин , продаваемый под названием бренда Apokyn, среди прочих, является типом апорфина , обладающего активностью в качестве не селективного агониста дофамина , который активирует как D 2 , так и, в гораздо меньшей степени, D 1 -подобные рецепторы . [ 2 ] Он также действует как антагонист 5 -HT 2 и α-адренергических рецепторов с высокой аффинностью . Соединение исторически представляет собой продукт декомпозиции морфина, изготовленный путем кипячения морфина с концентрированной кислотой, отсюда -суффикс -морфин . Вопреки его названию, апоморфин на самом деле не содержит морфина или его скелета, а также не связывается с опиоидными рецепторами . Apo - prefix относится к тому, что он является производным морфина («[происходит] из морфина»).
Исторически, апоморфин был опробован для различных применений, в том числе как способ облегчить беспокойство и тягу к алкоголикам, эметику (для вызывания рвоты), для лечения стереотипов (повторное поведение) у животных на ферме и в последнее время при лечении эректильной дисфункции Полем В настоящее время апоморфин используется при лечении болезни Паркинсона . Это мощный эметик и не следует вводить без противорвозной, такой как домперидон . Эметические свойства апоморфина используются в ветеринарной медицине, чтобы вызвать терапевтическую эры , которые недавно принимали токсичные или инородные вещества.
Apomorphine was also used as a private treatment of heroin addiction, a purpose for which it was championed by the author William S. Burroughs. Burroughs and others claimed that it was a "metabolic regulator" with a restorative dimension to a damaged or dysfunctional dopaminergic system. Despite anecdotal evidence that this offers a plausible route to an abstinence-based mode, no clinical trials have ever tested this hypothesis. A recent study indicates that apomorphine might be a suitable marker for assessing central dopamine system alterations associated with chronic heroin consumption.[3] There is, however, no clinical evidence that apomorphine is an effective and safe treatment regimen for opiate addiction.[4]
Medical uses
[edit]Apomorphine is used in advanced Parkinson's disease intermittent hypomobility ("off" episodes), where a decreased response to an anti-Parkinson drug such as L-DOPA causes muscle stiffness and loss of muscle control.[5][6] While apomorphine can be used in combination with L-DOPA, the intention is usually to reduce the L-DOPA dosing, as by this stage the patient often has many of dyskinesias caused by L-DOPA and hypermobility periods.[7][8] When an episode sets in, the apomorphine is injected subcutaneously or applied sublingually,[9] and signs subside. It is used an average of three times a day.[7] Some people use portable mini-pumps that continuously infuse them with apomorphine, allowing them to stay in the "on" state and using apomorphine as an effective monotherapy.[8][10]
Contraindications
[edit]The main and absolute contraindication to using apomorphine is the concurrent use of adrenergic receptor antagonists; combined, they cause a severe drop in blood pressure and fainting.[7][6] Alcohol causes an increased frequency of orthostatic hypotension (a sudden drop in blood pressure when getting up), and can also increase the chances of pneumonia and heart attacks.[7] Dopamine antagonists, by their nature of competing for sites at dopamine receptors, reduce the effectiveness of the agonistic apomorphine.[7][6]
IV administration of apomorphine is highly discouraged, as it can crystallize in the veins and create a blood clot (thrombus) and block a pulmonary artery (pulmonary embolism).[7][6]
Side effects
[edit]Nausea and vomiting are common side effects when first beginning therapy with apomorphine;[11] antiemetics such as trimethobenzamide or domperidone, dopamine antagonists,[12] are often used while first starting apomorphine. Around 50% of people grow tolerant enough to apomorphine's emetic effects that they can discontinue the antiemetic.[6][7]
Other side effects include orthostatic hypotension and resultant fainting, sleepiness, dizziness, runny nose, sweating, paleness, and flushing. More serious side effects include dyskinesias (especially when taking L-DOPA), fluid accumulation in the limbs (edema), suddenly falling asleep, confusion and hallucinations, increased heart rate and heart palpitations, and persistent erections (priapism).[6][7][13] The priapism is caused by apomorphine increasing arterial blood supply to the penis. This side effect has been exploited in studies attempting to treat erectile dysfunction.[14]
Pharmacology
[edit]Mechanism of action
[edit]Apomorphine's R-enantiomer is an agonist of both D1 and D2 dopamine receptors, with higher activity at D2.[7][12] The members of the D2 subfamily, consisting of D2, D3, and D4 receptors, are inhibitory G protein–coupled receptors. The D4 receptor in particular is an important target in the signaling pathway, and is connected to several neurological disorders.[15] Shortage or excess of dopamine can prevent proper function and signaling of these receptors leading to disease states.[16]
Apomorphine improves motor function by activating dopamine receptors in the nigrostriatal pathway, the limbic system, the hypothalamus, and the pituitary gland.[17] It also increases blood flow to the supplementary motor area and to the dorsolateral prefrontal cortex (stimulation of which has been found to reduce the tardive dyskinesia effects of L-DOPA).[18][19] Parkinson's has also been found to have excess iron at the sites of neurodegeneration; both the (R)- and (S)-enantiomers of apomorphine are potent iron chelators and radical scavengers.[12][20]
Apomorphine also decreases the breakdown of dopamine in the brain (though it inhibits its synthesis as well).[21][22] It is an upregulator of certain neural growth factors,[23] in particular NGF but not BDNF, epigenetic downregulation of which has been associated with addictive behaviour in rats.[24][25]
Apomorphine causes vomiting by acting on dopamine receptors in the chemoreceptor trigger zone of the medulla; this activates the nearby vomiting center.[17][22][26]
Pharmacokinetics
[edit]While apomorphine has lower bioavailability when taken orally, due to not being absorbed well in the GI tract and undergoing heavy first-pass metabolism,[20][10] it has a bioavailability of 100% when given subcutaneously.[7][17] It reaches peak plasma concentration in 10–60 minutes. Ten to twenty minutes after that, it reaches its peak concentration in the cerebrospinal fluid. Its lipophilic structure allows it to cross the blood–brain barrier.[7][17]
Apomorphine possesses affinity for the following receptors (note that a higher Ki indicates a lower affinity):[27][28][29]
| Receptor | Ki (nM) | Action |
|---|---|---|
| D1 | 484 | (partial) agonista |
| D2 | 52 | partial agonist (IA = 79% at D2S; 53% at D2L) |
| D3 | 26 | partial agonist (IA = 82%) |
| D4 | 4.37 | partial agonist (IA = 45%) |
| D5 | 188.9 | (partial) agonista |
| aThough its efficacies at D1 and D5 are unclear, it is known to act as an agonist at these sites.[30] | ||
| Receptor | Ki (nM) | Action |
|---|---|---|
| 5-HT1A | 2,523 | partial agonist |
| 5-HT1B | 2,951 | no action |
| 5-HT1D | 1,230 | no action |
| 5-HT2A | 120 | antagonist |
| 5-HT2B | 132 | antagonist |
| 5-HT2C | 102 | antagonist |
| Receptor | Ki (nM) | Action |
|---|---|---|
| α1A-adrenergic | 1,995 | antagonist |
| α1B-adrenergic | 676 | antagonist |
| α1D-adrenergic | 64.6 | antagonist |
| α2A-adrenergic | 141 | antagonist |
| α2B-adrenergic | 66.1 | antagonist |
| α2C-adrenergic | 36.3 | antagonist |
It has a Ki of over 10,000 nM (and thus negligible affinity) for β-adrenergic, H1, and mACh.[2]
Apomorphine has a high clearance rate (3–5 L/kg/hr) and is mainly metabolized and excreted by the liver.[17] It is likely that while the cytochrome P450 system plays a minor role, most of apomorphine's metabolism happens via auto-oxidation, O-glucuronidation, O-methylation, N-demethylation, and sulfation.[7][17][22] Only 3–4% of the apomorphine is excreted unchanged and into the urine. The half-life is 30–60 minutes, and the effects of the injection last for up to 90 minutes.[7][8][17]
Toxicity depends on the route of administration; the LD50s in mice were 300 mg/kg for the oral route, 160 mg/kg for intraperitoneal, and 56 mg/kg intravenous.[31]
Chemistry
[edit]Properties
[edit]Apomorphine has a catechol structure similar to that of dopamine.[21]
Synthesis
[edit]Several techniques exist for the creation of apomorphine from morphine. In the past, morphine had been combined with hydrochloric acid at high temperatures (around 150 °C) to achieve a low yield of apomorphine, ranging anywhere from 0.6% to 46%.[32]
More recent techniques create the apomorphine in a similar fashion, by heating it in the presence of any acid that will promote the essential dehydration rearrangement of morphine-type alkaloids, such as phosphoric acid. The method then deviates by including a water scavenger, which is essential to remove the water produced by the reaction that can react with the product and lead to decreased yield. The scavenger can be any reagent that will irreversibly react with water such as phthalic anhydride or titanium chloride. The temperature required for the reaction varies based upon choice of acid and water scavenger. The yield of this reaction is much higher: at least 55%.[32]

History
[edit]The pharmacological effects of the naturally-occurring analog aporphine in the blue lotus (Nymphaea caerulea)[34] were known to the ancient Egyptians and Mayans,[35] with the plant featuring in tomb frescoes and associated with entheogenic rites. It is also observed in Egyptian erotic cartoons, suggesting that they were aware of its erectogenic properties.
The modern medical history of apomorphine begins with its synthesis by Arppe in 1845[36] from morphine and sulfuric acid, although it was named sulphomorphide at first. Matthiesen and Wright (1869) used hydrochloric acid instead of sulfuric acid in the process, naming the resulting compound apomorphine. Initial interest in the compound was as an emetic, tested and confirmed safe by London doctor Samuel Gee,[37] and for the treatment of stereotypies in farmyard animals.[38] Key to the use of apomorphine as a behavioural modifier was the research of Erich Harnack, whose experiments in rabbits (which do not vomit) demonstrated that apomorphine had powerful effects on the activity of rabbits, inducing licking, gnawing and in very high doses convulsions and death.
Treatment of alcoholism
[edit]Apomorphine was one of the earliest used pharmacotherapies for alcoholism. The Keeley Cure (1870s to 1900) contained apomorphine, among other ingredients, but the first medical reports of its use for more than pure emesis come from James Tompkins[39] and Charles Douglas.[40][41] Tompkins reported, after injection of 6.5 mg ("one tenth of a grain"):
In four minutes free emesis followed, rigidity gave way to relaxation, excitement to somnolence, and without further medication the patient, who before had been wild and delirious, went off into a quiet sleep.
Douglas saw two purposes for apomorphine:
[it can be used to treat] a paroxysm of dipsomania [an episode of intense alcoholic craving]... in minute doses it is much more rapidly efficient in stilling the dipsomaniac craving than strychnine or atropine… Four or even 3m [minim – roughly 60 microlitres] of the solution usually checks for some hours the incessant demands of the patient… when he awakes from the apomorphine sleep he may still be demanding alcohol, though he is never then so insistent as before. Accordingly it may be necessary to repeat the dose, and even to continue to give it twice or three times a day. Such repeated doses, however, do not require to be so large: 4 or even 3m is usually sufficient.
This use of small, continuous doses (1/30th of a grain, or 2.16 mg by Douglas) of apomorphine to reduce alcoholic craving comes some time before Pavlov's discovery and publication of the idea of the "conditioned reflex" in 1903. This method was not limited to Douglas; the Irish doctor Francis Hare, who worked in a sanatorium outside London from 1905 onward, also used low-dose apomorphine as a treatment, describing it as "the most useful single drug in the therapeutics of inebriety".[42] He wrote:
In (the) sanatorium it is used in three different sets of circumstances: (1) in maniacal or hysterical drunkenness: (2) during the paroxysm of dipsomania, in order to still the craving for alcohol; and (3) in essential insomnia of a special variety... [after giving apomorphine] the patient's mental condition is entirely altered. He may be sober: he is free from the time being from any craving from alcohol. The craving may return, however, and then it is necessary to repeat the injection, it may be several times at intervals of a few hours. These succeeding injections should be quite small, 3 to 6 min. being sufficient. Doses of this size are rarely emetic. There is little facial pallor, a sensation as of the commencement of sea-sickness, perhaps a slight malaise with a sudden subsidence of the craving for alcohol, followed by a light and short doze.
He also noted there appeared to be a significant prejudice against the use of apomorphine, both from the associations of its name and doctors being reluctant to give hypodermic injections to alcoholics. In the US, the Harrison Narcotics Tax Act made working with any morphine derivatives extremely hard, despite apomorphine itself not being an opiate.
In the 1950s the neurotransmitter dopamine was discovered in the brain by Katharine Montagu, and characterised as a neurotransmitter a year later by Arvid Carlsson, for which he would be awarded the Nobel Prize.[43] A. N. Ernst then discovered in 1965 that apomorphine was a powerful stimulant of dopamine receptors.[44] This, along with the use of sublingual apomorphine tablets, led to a renewed interest in the use of apomorphine as a treatment for alcoholism. A series of studies of non-emetic apomorphine in the treatment of alcoholism were published, with mostly positive results.[45][46][47][48][49] However, there was little clinical consequence.
Parkinson's disease
[edit]The use of apomorphine to treat "the shakes" was first suggested by Weil in France in 1884,[50] although seemingly not pursued until 1951.[51] Its clinical use was first reported in 1970 by Cotzias et al.,[52] although its emetic properties and short half-life made oral use impractical. A later study found that combining the drug with the antiemetic domperidone improved results significantly.[53] The commercialization of apomorphine for Parkinson's disease followed its successful use in patients with refractory motor fluctuations using intermittent rescue injections and continuous infusions.[54]
Aversion therapy
[edit]Aversion therapy in alcoholism had its roots in Russia in the early 1930s,[55] with early papers by Pavlov, Galant and Sluchevsky and Friken,[56] and would remain a strain in the Soviet treatment of alcoholism well into the 1980s. In the US a particularly notable devotee was Dr Voegtlin,[57] who attempted aversion therapy using apomorphine in the mid to late 1930s. However, he found apomorphine less able to induce negative feelings in his subjects than the stronger and more unpleasant emetic emetine.
In the UK, however, the publication of J. Y. Dent's (who later went on to treat Burroughs) 1934 paper "Apomorphine in the treatment of Anxiety States"[58] laid out the main method by which apomorphine would be used to treat alcoholism in Britain. His method in that paper is clearly influenced by the then-novel idea of aversion:
He is given his favourite drink, and his favourite brand of that drink ... He takes it stronger than is usual to him ... The small dose of apomorphine, one-twentieth of a grain [3.24 mg], is now given subcutaneously into his thigh, and he is told that he will be sick in a quarter of an hour. A glass of whisky and water and a bottle of whisky are left by his bedside. At six o'clock (four hours later) he is again visited and the same treatment is again administered ... The nurse is told in confidence that if he does not drink, one-fortieth [1.62 mg] of a grain of apomorphine should be injected during the night at nine o'clock, one o'clock, and five o'clock, but that if he drinks the injection should be given soon after the drink and may be increased to two hourly intervals. In the morning at about ten he is again given one or two glasses of whisky and water ... and again one-twentieth of a grain [3.24 mg] of apomorphine is injected ... The next day he is allowed to eat what he likes, he may drink as much tea as he likes ... He will be strong enough to get up and two days later he leaves the home.
However, even in 1934 he was suspicious of the idea that the treatment was pure conditioned reflex – "though vomiting is one of the ways that apomorphine relives the patient, I do not believe it to be its main therapeutic effect." – and by 1948 he wrote:[4]
It is now twenty-five years since I began treating cases of anxiety and alcoholism with apomorphine, and I read my first paper before this Society fourteen years ago. Up till then I had thought, and, unfortunately, I said in my paper, that the virtue of the treatment lay in the conditioned reflex of aversion produced in the patient. This statement is not even a half truth… I have been forced to the conclusion that apomorphine has some further action than the production of a vomit.
This led to his development of lower-dose and non-aversive methods, which would inspire a positive trial of his method in Switzerland by Dr Harry Feldmann[59] and later scientific testing in the 1970s, some time after his death. However, the use of apomorphine in aversion therapy had escaped alcoholism, with its use to treat homosexuality leading to the death of a British Army Captain Billy Clegg Hill in 1962,[60] helping to cement its reputation as a dangerous drug used primarily in archaic behavioural therapies.
Opioid addiction
[edit]In his Deposition: Testimony Concerning a Sickness in the introduction to later editions of Naked Lunch (first published in 1959), William S. Burroughs wrote that apomorphine treatment was the only effective cure to opioid addiction he has encountered:
The apomorphine cure is qualitatively different from other methods of cure. I have tried them all. Short reduction, slow reduction, cortisone, antihistamines, tranquilizers, sleeping cures, tolserol, reserpine. None of these cures lasted beyond the first opportunity to relapse. I can say that I was never metabolically cured until I took the apomorphine cure... The doctor, John Yerbury Dent, explained to me that apomorphine acts on the back brain to regulate the metabolism and normalize the blood stream in such a way that the enzyme stream of addiction is destroyed over a period of four to five days. Once the back brain is regulated apomorphine can be discontinued and only used in case of relapse.
He goes on to lament the fact that as of his writing, little to no research has been done on apomorphine or variations of the drug to study its effects on curing addiction, and perhaps the possibility of retaining the positive effects while removing the side effect of vomiting.
Despite his claims throughout his life, Burroughs never really cured his addiction and was back to using opiates within years of his apomorphine "cure".[61] However, he insisted on apomorphine's effectiveness in several works and interviews.[citation needed]
Society and culture
[edit]- Apomorphine has a vital part in Agatha Christie's detective story Sad Cypress.
- The 1965 Tuli Kupferberg song "Hallucination Horrors" recommends apomorphine at the end of each verse as a cure for hallucinations brought on by a humorous variety of intoxicants; the song was recorded by The Fugs and appears on the album Virgin Fugs.
Research
[edit]There is renewed interest in the use of apomorphine to treat addiction, in both smoking cessation[62] and alcoholism.[63] As the drug is known to be reasonably safe for use in humans, it is a viable target for repurposing.

Apomorphine has been researched as a possible treatment for erectile dysfunction and female hypoactive sexual desire disorder, though its efficacy has been limited.[14][64] Nonetheless, it was under development as a treatment for erectile dysfunction by TAP Pharmaceuticals under the brand name Uprima. In 2000, TAP withdrew its new drug application after an FDA review panel raised questions about the drug's safety, due to many clinical trial subjects fainting after taking the drug.[65]
Alzheimer's disease
[edit]Apomorphine is reported to be an inhibitor of amyloid beta protein fiber formation, whose presence is a hallmark of Alzheimer's disease, and a potential therapeutic under the amyloid hypothesis.[66]
Alternative administration routes
[edit]Two routes of administration are currently clinically utilized: subcutaneous (either as intermittent injections or continuous infusion) and sublingual. Other non-invasive administration routes were investigated as a substitute for parenteral administration, reaching different preclinical and clinical stages. These include: peroral,[67] nasal,[68][69][70][71] pulmonary,[72] transdermal,[73] rectal,[74][75] and buccal,[76][77] as well as iontophoresis methods.[78]
Veterinary use
[edit]Apomorphine is used to inducing vomiting in dogs after ingestion of various toxins or foreign bodies. It can be given subcutaneously, intramuscularly, intravenously, or, when a tablet is crushed, in the conjunctiva of the eye.[79][80] The oral route is ineffective, as apomorphine cannot cross the blood–brain barrier fast enough, and blood levels don't reach a high enough concentration to stimulate the chemoreceptor trigger zone.[79] It can remove around 40–60% of the contents in the stomach.[81]
One of the reasons apomorphine is a preferred drug is its reversibility:[82] in cases of prolonged vomiting, the apomorphine can be reversed with dopamine antagonists like the phenothiazines (for example, acepromazine). Giving apomorphine after giving acepromazine, however, will no longer stimulate vomiting, because apomorphine's target receptors are already occupied.[79] An animal who undergoes severe respiratory depression due to apomorphine can be treated with naloxone.[79][80]
Apomorphine does not work in cats, who have too few dopamine receptors.[79]
Related compounds
[edit]Mdo-npa, the methylenedioxy analog of apomorphine, has greater bioavailability and a longer duration of action.[citation needed]
References
[edit]- ^ "Movapo Pod (Stada Pharmaceuticals Australia Pty Ltd)". Therapeutic Goods Administration (TGA). 13 September 2024. Retrieved 15 September 2024.
- ^ Jump up to: a b Millan MJ, Maiofiss L, Cussac D, Audinot V, Boutin JA, Newman-Tancredi A (November 2002). "Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. I. A multivariate analysis of the binding profiles of 14 drugs at 21 native and cloned human receptor subtypes". The Journal of Pharmacology and Experimental Therapeutics. 303 (2): 791–804. doi:10.1124/jpet.102.039867. PMID 12388666. S2CID 6200455.
- ^ Guardia J, Casas M, Prat G, Trujols J, Segura L, Sánchez Turet M (October 2002). "The apomorphine test: a biological marker for heroin dependence disorder?". Addiction Biology. 7 (4): 421–426. doi:10.1080/1355621021000006206. PMID 14578019. S2CID 32386793.
- ^ Jump up to: a b Dent JY (1949). "Apomorphine Treatment of Addiction". British Journal of Addiction to Alcohol & Other Drugs. 46 (1): 15–28. doi:10.1111/j.1360-0443.1949.tb04502.x.
- ^ "Apomorphine Uses, Side Effects & Warnings". Drugs.com. Retrieved 27 February 2018.
- ^ Jump up to: a b c d e f Clayton BD, Willihnganz M (2016). Basic Pharmacology for Nurses – E-Book. Elsevier Health Sciences. pp. 210–211. ISBN 978-0-323-37697-6.
- ^ Jump up to: a b c d e f g h i j k l m "Apomorphine Hydrochloride Monograph for Professionals". Drugs.com. Retrieved 26 February 2018.
- ^ Jump up to: a b c Chaudhuri KR, Clough C (February 1998). "Subcutaneous apomorphine in Parkinson's disease". BMJ. 316 (7132): 641. doi:10.1136/bmj.316.7132.641. PMC 1112674. PMID 9522772.
- ^ Paton DM (January 2021). "Apomorphine hydrochloride: a sublingual tablet for the OFF episodes in Parkinson's disease". Drugs of Today. 57 (1): 5–16. doi:10.1358/dot.2021.57.1.3211618. PMID 33594386. S2CID 231945531.
- ^ Jump up to: a b Schapira AH, Olanow CW (2005). Principles of Treatment in Parkinson's Disease (illustrated ed.). Elsevier Health Sciences. p. 35. ISBN 978-0-7506-5428-9.
- ^ Dressler D (June 2005). "Apomorphin bei der Behandlung des Morbus Parkinson" [Apomorphine in the treatment of Parkinson's Disease]. Der Nervenarzt (in German). 76 (6): 681–689. doi:10.1007/s00115-004-1830-4. PMID 15592807. S2CID 19617827.
- ^ Jump up to: a b c Youdim MB, Gassen M, Gross A, Mandel S, Grünblatt E (2000). "Iron chelating, antioxidant and cytoprotective properties of dopamine receptor agonist; apomorphine". In Mizuno Y, Calne DB, Horowski R, Poewe W, Riederer P, Youdim MB (eds.). Advances in Research on Neurodegeneration, vol. 7. Seventh International Winter Conference on Neurodegeneration and Neuroinflammation. Karuizawa, Nagano, Japan. January 20–22, 1999. Journal of Neural Transmission. Supplementum. No. 58. Springer Science & Business Media. pp. 83–96. doi:10.1007/978-3-7091-6284-2_7. ISBN 978-3-211-83485-5. PMID 11128615.
- ^ "Apomorphine". Medline Plus. US National Library of Medicine. 15 June 2017. Retrieved 26 February 2018.
- ^ Jump up to: a b Porst H, Buvat J (2008). Standard Practice in Sexual Medicine. John Wiley & Sons. p. 77. ISBN 978-1-4051-7872-3.
- ^ Ptácek R, Kuzelová H, Stefano GB (September 2011). "Dopamine D4 receptor gene DRD4 and its association with psychiatric disorders". Medical Science Monitor. 17 (9): RA215–RA220. doi:10.12659/MSM.881925. PMC 3560519. PMID 21873960.
- ^ Stacy M, Silver D (2008). "Apomorphine for the acute treatment of "off" episodes in Parkinson's disease". Parkinsonism & Related Disorders. 14 (2): 85–92. doi:10.1016/j.parkreldis.2007.07.016. PMID 18083605.
- ^ Jump up to: a b c d e f g U.S. National Library of Medicine. "Apomorphine". PubChem. Retrieved 26 February 2018.
- ^ Lewitt P, Oertel WH (1999). Parkinson's Disease: The Treatment Options. CRC Press. p. 22. ISBN 978-1-85317-379-0.
- ^ Rektorová I, Sedláčková S, Telečka S, Hlubočky A, Rektor I (2008). "Dorsolateral prefrontal cortex: a possible target for modulating dyskinesias in Parkinson's disease by repetitive transcranial magnetic stimulation". International Journal of Biomedical Imaging. 2008: 372125. doi:10.1155/2008/372125. PMC 2233877. PMID 18274665.
- ^ Jump up to: a b Galvez-Jimenez M (2013). Scientific Basis for the Treatment of Parkinson's Disease (2nd ed.). CRC Press. p. 195. ISBN 978-0-203-33776-9.
- ^ Jump up to: a b Iversen L (2012). Biogenic Amine Receptors. Springer Science & Business Media. p. 238. ISBN 978-1-4684-8514-1.
- ^ Jump up to: a b c Advances in Pharmacology and Chemotherapy. Vol. 15. Silvio Garattini, A. Goldin, F. Hawking, Irwin J. Kopin. Academic Press. 1978. pp. 27, 93, 96. ISBN 978-0-08-058106-4.
{{cite book}}: CS1 maint: others (link) - ^ Ohta M, Mizuta I, Ohta K, Nishimura M, Mizuta E, Hayashi K, et al. (May 2000). "Apomorphine up-regulates NGF and GDNF synthesis in cultured mouse astrocytes". Biochemical and Biophysical Research Communications. 272 (1): 18–22. doi:10.1006/bbrc.2000.2732. PMID 10872797.
- ^ McGeary JE, Gurel V, Knopik VS, Spaulding J, McMichael J (October 2011). "Effects of nerve growth factor (NGF), fluoxetine, and amitriptyline on gene expression profiles in rat brain". Neuropeptides. 45 (5): 317–322. doi:10.1016/j.npep.2011.06.002. PMID 21820738. S2CID 38444849.
- ^ Heberlein A, Muschler M, Frieling H, Behr M, Eberlein C, Wilhelm J, et al. (Май 2013). «Эпигенетическая регуляция фактора роста нервов во время отмены алкоголя». Биология зависимости . 18 (3): 508–510. doi : 10.1111/j.1369-1600.2010.00307.x . PMID 21392176 . S2CID 20317993 .
{{cite journal}}: CS1 maint: overridden setting (link) - ^ Riviere JE, Papich MG (2009). Ветеринарная фармакология и терапия . Джон Уайли и сыновья. п. 318. ISBN 978-0-8138-2061-3 .
- ^ Рот Бл , Дрискол Дж. (12 января 2011 г.). "PDSP K I Database" . Психоактивная программа скрининга лекарств (PDSP) . Университет Северной Каролины в Чапел -Хилл и Национальный институт психического здоровья США . Получено 1 июля 2014 года . Примечание. Используются значения для людей. Если для людей указано более одного значения, их среднее значение используется.
- ^ Newman-Tancredi A, Cussac D, Audinot V, Nicolas JP, De Ceuninck F, Boutin JA, et al. (Ноябрь 2002). «Дифференциальные действия антипаркинсонов агентов в нескольких классах моноаминергического рецептора. II. Свойства агониста и антагониста в подтипах дофамина D 2 -рецептора и α 1 /α 2 -адренорецептор». Журнал фармакологии и экспериментальной терапии . 303 (2): 805–814. doi : 10.1124/jpet.102.039875 . PMID 12388667 . S2CID 35238120 .
- ^ Newman-Tancredi A, Cussac D, Quentric Y, Touzard M, Verrièle L, Carpentier N, et al. (Ноябрь 2002). «Дифференциальное действие антипаркинсонов агентов в нескольких классах моноаминергического рецептора. III. Свойства агониста и антагониста в серотонине, 5-HT 1 и 5-HT 2 , рецепторные подтипы». Журнал фармакологии и экспериментальной терапии . 303 (2): 815–822. doi : 10.1124/jpet.102.039883 . PMID 12388668 . S2CID 19260572 .
- ^ Sieh GC, Hollingsworth PR, Martino B, Chang R, Terranova MA, O'Neill AB, et al. (Январь 2004 г.). «Центральные механизмы, регулирующие эрекцию полового члена у сознательных крыс: дофаминергические системы, связанные с эффектом апоморфина». Журнал фармакологии и экспериментальной терапии . 308 (1): 330–338. doi : 10.1124/jpet.103.057455 . PMID 14569075 . S2CID 7485959 .
{{cite journal}}: Cs1 maint: переопределенная настройка ( ссылка ) - ^ Льюис С.Р. Р.Дж. (2004). Опасные свойства Саксорога промышленных материалов (11 изд.). Wiley, John & Sons, Incorporated. п. 287. ISBN 978-0471476627 .
- ^ Jump up to: а беременный Гурусами Н. «Процесс создания апоморфина и апокодеина» .
- ^ Bentley KW (24 апреля 2014 г.). Изохинолиновые алкалоиды: курс по органической химии . Elsevier, 2014. С. 118–120. ISBN 978-1483152233 .
- ^ Poklis JL, Mulder HA, Halquist MS, Wolf CE, Poklis A, Мир MR (июль 2017 г.). «Синий цветок лотоса ( Nymphea Caerulea ) смола, используемая в новом типе электронных сигарет, повторно разбираемого распылителя» . Журнал психоактивных лекарств . 49 (3): 175–181. doi : 10.1080/02791072.2017.1290304 . PMC 5638439 . PMID 28266899 .
- ^ Бертол Е., Финчи В., Карх С.Б., Мари Ф., Риццо I (февраль 2004 г.). « Nymphaea Cults в древнем Египте и Новом Свете: урок эмпирической фармакологии» . Журнал Королевского общества медицины . 97 (2): 84–85. doi : 10.1177/014107680409700214 . PMC 1079300 . PMID 14749409 .
- ^ Таба П., Лис А., Стерн Г. (2013). «Эрих Харнак (1852–1915) и короткая история апоморфина» . Европейская неврология . 69 (6): 321–324. doi : 10.1159/000346762 . PMID 23549143 .
- ^ Джив (1869). «О действии новой органической базы, апоморфия». Труды Клинического общества Лондона . 2 : 166–169.
- ^ Фесер Дж (1873). «Недавний отдых: 1. Апоморфиновая гидрохлоратум» [самые недавно использованные лекарства: 1. Апоморфины гидрохлорид]. Z практикует Veterinairwiss : 302-306.
- ^ Tompkins J (1899). «Апоморфин в острого алкогольного бреда». Медицинская карта .
- ^ «Апоморфин как гипнотический». Lancet . 155 (3998): 1083. апрель 1900 года. DOI : 10.1016/S0140-6736 (01) 70565-X .
- ^ Дуглас CJ (1899). «Изъятие алкоголя в бредовом дроби». Нью -Йоркский медицинский журнал : 626.
- ^ Заяц F (1912). Об алкоголизме; его клинические аспекты и лечение . Лондон: Черчилль.
- ^ Бенес FM (январь 2001 г.). «Карлссон и открытие дофамина». Тенденции в фармакологических науках . 22 (1): 46–47. doi : 10.1016/s0165-6147 (00) 01607-2 . PMID 11165672 .
- ^ Эрнст А.М. (май 1965). «Связь между действием дофамина и апоморфина и их ометилированными производными на ЦНС». Психофармакологии . 7 (6): 391–399. doi : 10.1007/bf00402361 . PMID 5831877 . S2CID 7445311 .
- ^ Мойнихан Н.Х. (август 1965 г.). «Лечение алкоголизма в общей практике». Практикующий . 195 : 223–227. PMID 14328866 .
- ^ Carlsson C, Johansson PR, Gullberg B (май 1977). «Двойное слепое исследование по пересечению: апоморфин/плацебо при хронических алкоголиков». Международный журнал клинической фармакологии и биофармедии . 15 (5): 211–213. PMID 326687 .
- ^ Халворсен К.А., Мартенсен-Ларсен О (апрель 1978 г.). «Апоморфин возродился: укрепленный, длительный и улучшенный терапевтический эффект». Международный журнал зависимости . 13 (3): 475–484. doi : 10.3109/10826087809045262 . PMID 352969 .
- ^ Дженсен С.Б., Кристофферсен К.Б., Норрегаард А (декабрь 1977 г.). «Апоморфин в амбулаторном лечении алкогольного опьянения и воздержания: двойное слепое исследование». Британский журнал зависимости от алкоголя и других наркотиков . 72 (4): 325–330. doi : 10.1111/j.1360-0443.1977.tb00699.x . PMID 341937 .
- ^ Schlatter EK, Lal S (июнь 1972 г.). «Лечение алкоголизма с помощью перорального апоморфинного метода Дента». Ежеквартальный журнал исследований по алкоголю . 33 (2): 430–436. doi : 10.15288/QJSA.1972.33.430 . PMID 5033142 .
- ^ Вейл Э (1884). «Апоморфин при определенных нервных расстройствах» [на апоморфине в определенных нервных коктейлях]. Лион Мед (по -французски). 48 : 411–419.
- ^ Schwab RS, Amador LV, Lettvin JY (1951). «Апоморфин при болезни Паркинсона». Сделки Американской неврологической ассоциации . 56 : 251–253. PMID 14913646 .
- ^ Cotzias GC, Papavasiliou PS, Fehling C, Kaufman B, Mena I (январь 1970 г.). «Сходство между неврологическими эффектами L-допа и апоморфином». Новая Англия Журнал медицины . 282 (1): 31–33. doi : 10.1056/nejm197001012820107 . PMID 4901383 .
- ^ Corsini Gu, Del Zompo M, Gessa GL, Mangoni A (май 1979). «Терапевтическая эффективность апоморфина в сочетании с внецеребральным ингибитором дофаминовых рецепторов при болезни Паркинсона». Лансет . 1 (8123): 954–956. doi : 10.1016/s0140-6736 (79) 91725-2 . PMID 87620 . S2CID 43526111 .
- ^ Stibe CM, Lees AJ, Kempster PA, Stern Gm (февраль 1988 г.). «Подкожный апоморфин у паркинсонианских колебаний». Лансет . 1 (8582): 403–406. doi : 10.1016/s0140-6736 (88) 91193-2 . PMID 2893200 . S2CID 35208453 .
- ^ Бан Т.А. (2008). Кондиционирующее поведение и психиатрия . New Brunswick [NJ]: AldineTransaction. ISBN 978-0-202-36235-9 Полем OCLC 191318001 .
- ^ Raikhel EA (2016). Основные привычки: лечение алкоголизма в постсоветской клинике . Итака. ISBN 9781501703133 Полем OCLC 965905763 .
{{cite book}}: CS1 Maint: местоположение отсутствует издатель ( ссылка ) - ^ Lemere F, Voegtlin WL (июнь 1950 г.). «Оценка отвращения к обращению с алкоголизмом». Ежеквартальный журнал исследований по алкоголю . 11 (2): 199–204. doi : 10.15288/QJSA.1950.11.199 . PMID 15424345 .
- ^ Дент Джи (1 октября 1934 г.). «Апоморфин в лечении состояний тревоги, с особой ссылкой на алкоголизм*». Британский журнал неопровержимости . 32 (2): 65–88. doi : 10.1111/j.1360-0443.1934.tb05016.x . ISSN 1360-0443 .
- ^ De Morsier G, Feldmann H (1952). «[Апоморфиновая терапия алкоголизма; доклад о 500 случаях]». Швейцарский архив неврологии и психиатрии. Архив Suisses de Neurology и De Psychiatry. Archivio Svizzero di Neurologia e Psichiatria . 70 (2): 434–440. PMID 13075975 .
- ^ «Гей -несправедливость» была широко распространена » . 12 сентября 2009 г. Получено 24 января 2018 года .
- ^ Бирмингем Дж (2 ноября 2009 г.). «Уильям Берроуз и история героина» . Realitystudio .
- ^ Моралес Росадо Дж.А., двоюродный брат М.А., Эбберт Джо, Кли Эв (декабрь 2015 г.). «Критический обзор перепрофилирования апоморфина для прекращения курения». Анализ и технологии разработки лекарств . 13 (10): 612–622. doi : 10.1089/adt.2015.680 . PMID 26690764 .
- ^ «Апоморфин - забытое лечение алкоголизма» . апоморфин.info . Получено 24 января 2018 года .
- ^ Ишак WW (2017). Учебник клинической сексуальной медицины . Спрингер. п. 388. ISBN 978-3-319-52539-6 .
- ^ «Эбботт снимает применение на таблетку для импотенции» . Bloomberg News через New York Times . 1 июля 2000 года.
- ^ Лашуэль Х.А., Хартли Д.М., Балахане Д., Аггарвал А., Тейхберг С., Каллавей Д.Джея (ноябрь 2002 г.). «Новый класс ингибиторов образования амилоид-бета-фибрилл. Последствия для механизма патогенеза при болезни Альцгеймера» . Журнал биологической химии . 277 (45): 42881–42890. doi : 10.1074/jbc.m206593200 . PMID 12167652 .
- ^ Borkar N, Holm R, Yang M, Müllertz A, Mu H (ноябрь 2016 г.). «In vivo оценка липидных составов для пероральной доставки апоморфина и его пролекарства в диэстер». Международный журнал фармацевтики . 513 (1–2): 211–217. doi : 10.1016/j.ijpharm.2016.09.024 . PMID 27615708 .
- ^ Netsombooon K, Partenhauser A, Roherer J, Elli Sündermann N, Prüfert F, Sighain W, et al. (Декабрь 2016 г.). «Предварительные тиомеры для интраназальной доставки апоморфина: in vitro и in vivo оценка». Европейский журнал фармацевтики и биофармацевтики . 109 : 35–42. doi : 10.1016/j.ejpb.2016.09.004 . PMID 27615996 .
{{cite journal}}: Cs1 maint: переопределенная настройка ( ссылка ) - ^ Kapoor R, Turjanski N, Frankel J, Kleedorfer B, Lees A, Stern G, et al. (Ноябрь 1990). «Интраназальный апоморфин: новое лечение при болезни Паркинсона» . Журнал неврологии, нейрохирургии и психиатрии . 53 (11): 1015. doi : 10.1136/jnnp.53.11.1015 . PMC 488291 . PMID 2283516 .
{{cite journal}}: Cs1 maint: переопределенная настройка ( ссылка ) - ^ Сэм Э., Джанжан А.П., Малоти Дж. М., Вербеке Н (март 1995 г.). «Апоморфиновая фармакокинетика в паркинсонизме после интраназального и подкожного применения». Европейский журнал по метаболизму наркотиков и фармакокинетики . 20 (1): 27–33. doi : 10.1007/bf03192285 . PMID 7588990 . S2CID 7126130 .
- ^ Ikechukwu Ugwoke M, Kaufmann G, Verbeke N, Kinget R (июль 2000 г.). «Интраназальная биодоступность апоморфина из систем доставки лекарств на основе карбоксиметилцеллюлозы». Международный журнал фармацевтики . 202 (1–2): 125–131. doi : 10.1016/s0378-5173 (00) 00434-8 . PMID 10915935 .
- ^ Гроссет К.А., Малек Н., Морган Ф., Гроссет Д.Г. (сентябрь 2013 г.). «Вдыхаемый апоморфин сухого порошка (VR040) для« отключенных »периодов при болезни Паркинсона: в двойной слепой дозе в рамках двойной слепой дозы» . Acta Neurologica Scandinavica . 128 (3): 166–171. doi : 10.1111/ane.12107 . PMID 23527823 . S2CID 22189634 .
- ^ Priano L, Albani G, Brioschi A, Calderoni S, Lopiano L, Rizzone M, et al. (Август 2004 г.). «Трансдермальное апоморфиновое проникновение от микроэмульсий: новое лечение при болезни Паркинсона». Движение расстройства . 19 (8): 937–942. doi : 10.1002/mds.20054 . HDL : 2318/41858 . PMID 15300660 . S2CID 28397399 .
{{cite journal}}: Cs1 maint: переопределенная настройка ( ссылка ) - ^ Ван Лаар Т., Янсен Эн, Эссинк А.В., Руттен В.Дж., Ниф С (август 1992). «Апоморфин прямой кишки: новый метод лечения при болезни Паркинсона» . Журнал неврологии, нейрохирургии и психиатрии . 55 (8): 737–738. doi : 10.1136/jnnp.55.8.737-A . PMC 489221 . PMID 1527553 .
- ^ Ван Лаар Т., Янсен Эн, Ниф С, Данхоф М., Роос Р.А. (июль 1995 г.). «Фармакокинетика и клиническая эффективность ректального апоморфина у пациентов с болезнью Паркинсона: исследование пяти различных суппозиторий». Движение расстройства . 10 (4): 433–439. doi : 10.1002/mds.870100405 . PMID 7565822 . S2CID 30947265 .
- ^ Itin C, Komargodski R, Barasch D, Domb AJ, Hoffman A (апрель 2021 г.). «Длительная доставка апоморфина через слизистую оболочку щека, в направлении неинвазивного метода устойчивого введения при болезни Паркинсона: исследования in vivo у свиней» . Журнал фармацевтических наук . 110 (4): 1824–1833. doi : 10.1016/j.xphs.2020.12.010 . ISSN 0022-3549 . PMID 33333142 . S2CID 229317834 .
- ^ Itin C, Komargodski R, Domb AJ, Hoffman A (сентябрь 2020 г.). «Контролируемая доставка апоморфина через слизистую оболочку щека, к неинвазивному методу введения при болезни Паркинсона: доклиническое механистическое исследование». Журнал фармацевтических наук . 109 (9): 2729–2734. doi : 10.1016/j.xphs.2020.05.017 . PMID 32497595 . S2CID 219331493 .
- ^ Li GL, De Vries JJ, Van Steeg TJ, Van Den Bussche H, Maas HJ, Reeuwijk HJ, et al. «Трансдермальная ионтофоретическая доставка или апоморфин у пациентов улучшились в предварительной обработке поверхностно -активного вещества». Журнал контролируемого выпуска . 101 (1–3): 199-208. Doi : 10.1016/j.jofrel.2004.09.011 . PMID 15588905 .
{{cite journal}}: Cs1 maint: переопределенная настройка ( ссылка ) - ^ Jump up to: а беременный в дюймовый и Билл Р.Л. (2016). Клиническая фармакология и терапия для ветеринарных техников-электронная книга . Elsevier Health Sciences. п. 94. ISBN 978-0-323-44402-6 .
- ^ Jump up to: а беременный Хан С.Н., Хузер С.Б. (2012). Общие токсикологические проблемы у мелких животных, проблема ветеринарных клиник: практика мелких животных-электронная книга . Elsevier Health Sciences. п. 310. ISBN 978-1-4557-4325-4 .
- ^ Plumb DC (2011). «Апоморфин». Справочник ветеринарного наркотиков Plumb (7 -е изд.). Стокгольм, Висконсин: Уайли. С. 77–79. ISBN 978-0-470-95964-0 .
- ^ Петерсон я, Талкотт П.А. (2006). Токсикология мелких животных . Elsevier Health Sciences. п. 131. ISBN 978-0-7216-0639-2 .